Matchless Ammonia Gas Reacts With Hydrogen Chloride Gas Equation
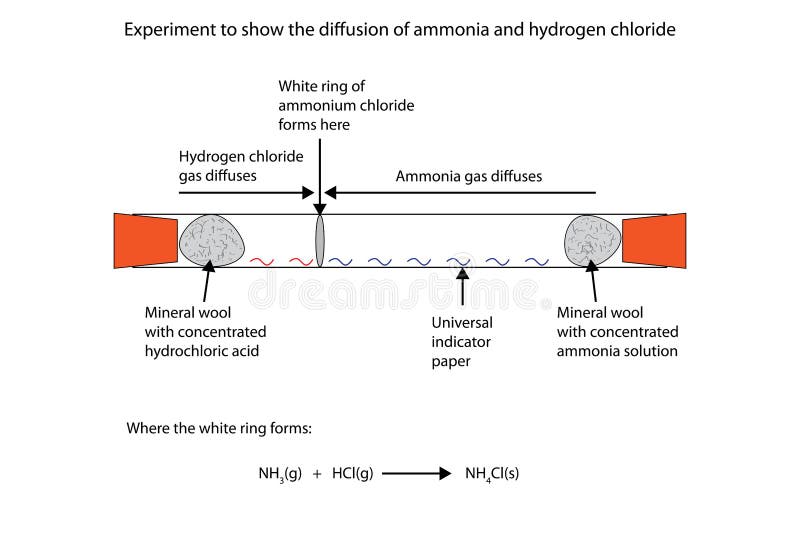
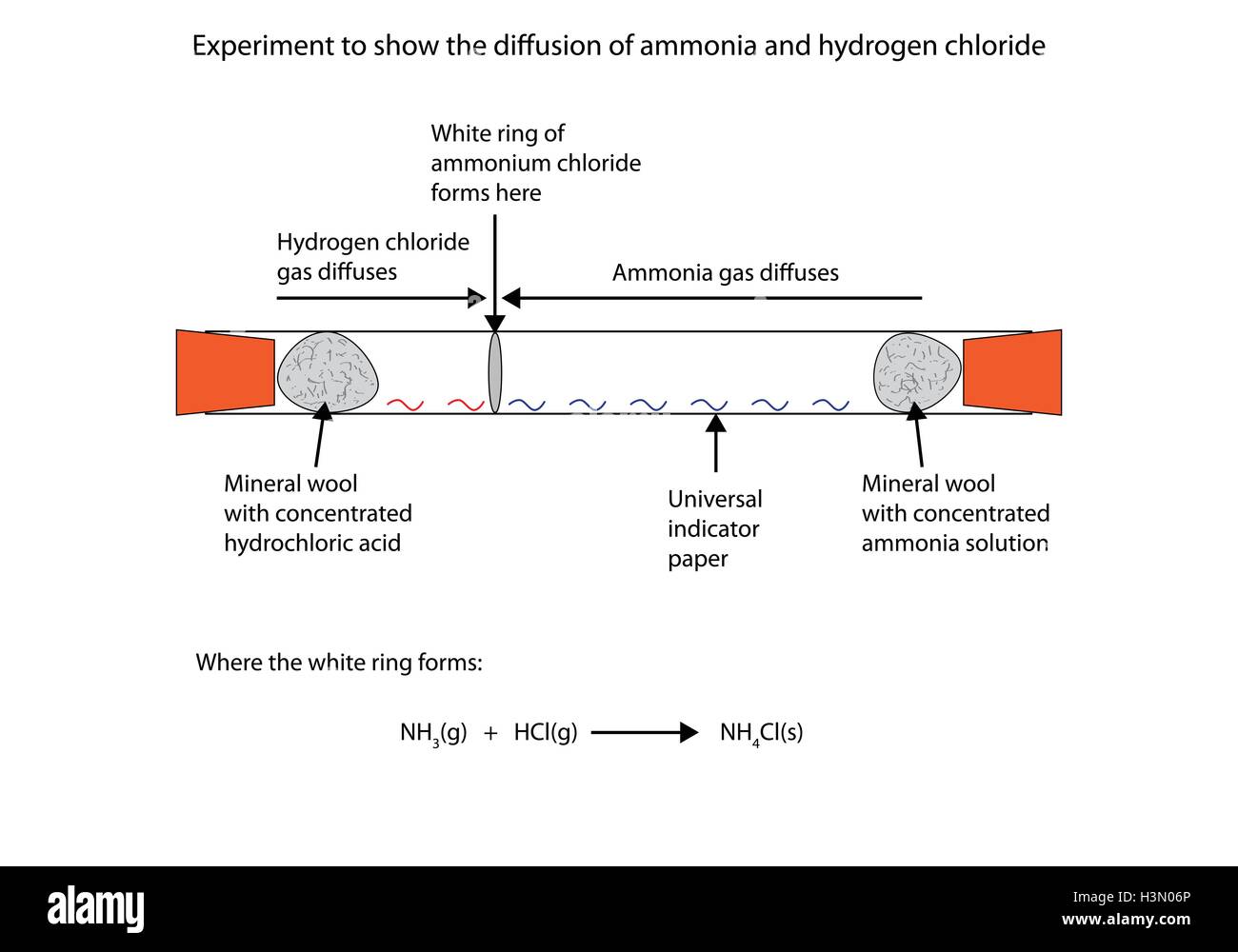
2 stoppered Erlenmeyer flasks with ammonia and hydrogen chloride gases are opened and the product of the reaction ammonium chloride white smoke will appea.
Ammonia gas reacts with hydrogen chloride gas equation. Ii How many grams of N H 3 C l will be formed when the stopper is opened. Write the formula equation for the following reacüon. It is a combination reaction.
Translate the following statements into chemical equations and then balance them. NH 3 g HCl g NH 4 Cl s Two 250 L flasks at 300 o C are connected by a stopcock as shown in the drawing One flask contains 560g NH 3 g and the other contains 460 g HCl g. Write the formula equation for the following reaction.
Solid magnesium carbonate reacts with a solution of hydrochloric acid. You could write this equation in two ways. In the late eighteenth century Priestley prepared ammonia by reacting mathrmHNO_3g with hydrogen gas.
Here only chlorine atoms should be considered. Concentrated hydrochloric acid gives off hydrogen chloride gas. Is window cleaner an acid or base acid.
Suppose we react an excess of magnesium metal with 132 mL of a 300 M solution of hydrochloric acid and collect all of the hydrogen in a. When heated calcium carbonate CaC03 decomposes to form calcium oxide and carbon dioxide. Most window cleaners are basic.
The reaction is exothermic. Solid ammonium chloride is produced. D Potassium metal react with water.