Ace Ammonia Reacts With Hcl
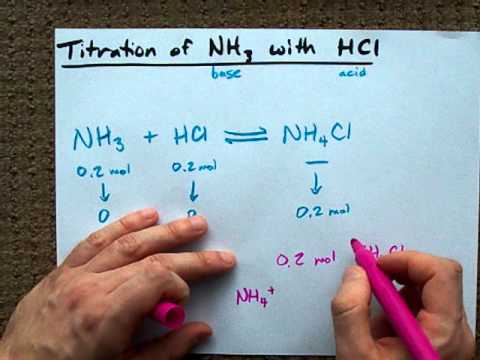
NH3g HCl g NH4Cls.
Ammonia reacts with hcl. What happens when you mix HCl and Na2CO3. Ethanoic acid reacts with ammonia in exactly the same way as any other acid does. The reaction equation between ammonia NH3 and hydrochloric acid HCl is written as follows.
First ammonia reacts with chlorine and produce nitrogen gas and hydrogen chloride vapor. It transfers a hydrogen ion to the lone pair on the nitrogen of the ammonia and forms an ammonium ion. NH3aq HClaq NH4Claq NH4OHaq HClaq NH4Claq H2Ol.
They neutralise each other if the quantities are right you end up with a neutral ionic solution of ammonium chloride. This creates an electron-adorned ammonium chloride an. NH 4 Cl NH 3 HCl Ammonium chloride reacts with a strong base like sodium hydroxide to release ammonia gas.
How much hydrochloric acid must be added to react completely. Problem Details Classify the reaction. Hydrochloric acid reacts with ammonia to form ammonium chloride a salt.
What happens when ammonia reacts with hydrochloric acid. Part of NCSSM CORE collection. This video shows the gas phase acid base reaction of Ammonia and Hydrochloric Acid.
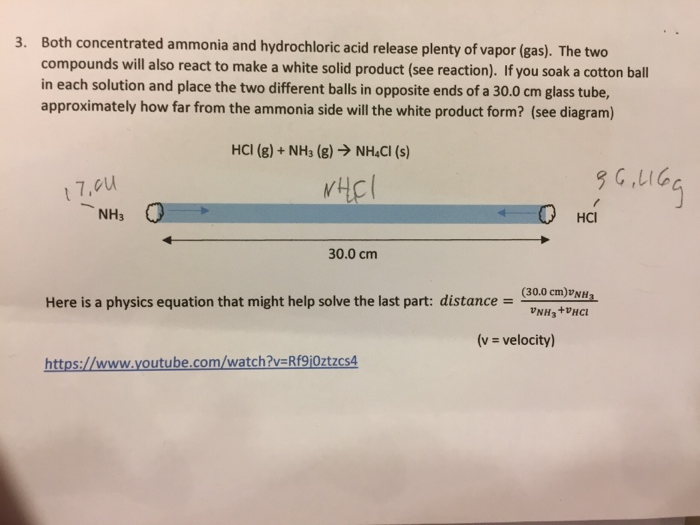
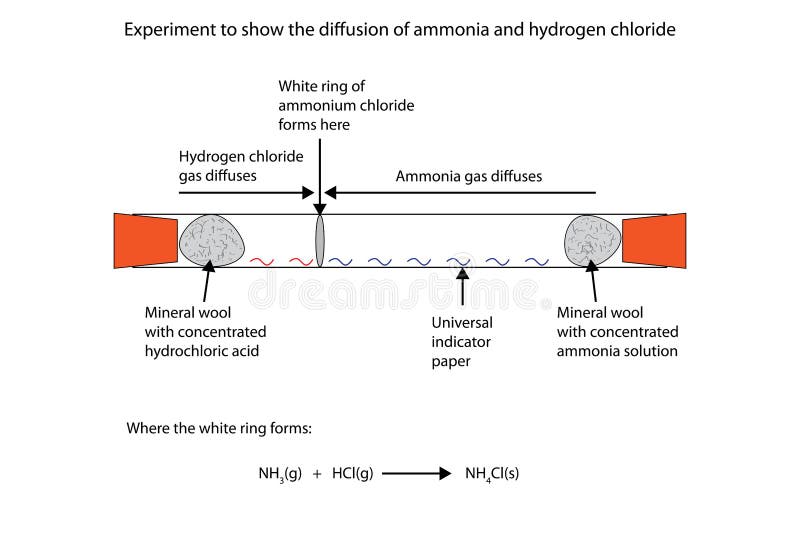
Ammonia and hydrogen chloride Place concentrated ammonia solution on a pad in one end of a tube and concentrated hydrochloric acid on a pad at the other and watch as the two gases diffuse far enough to meet and form a ring of solid ammonium chloride This demonstration is best performed in a fume cupboard. HCl H C l reacts with both the ammonia and ammonium chloride so the concentration of both decreases. If you mix together a solution of ethanoic acid and a solution of ammonia you will get a colorless solution of ammonium ethanoate.