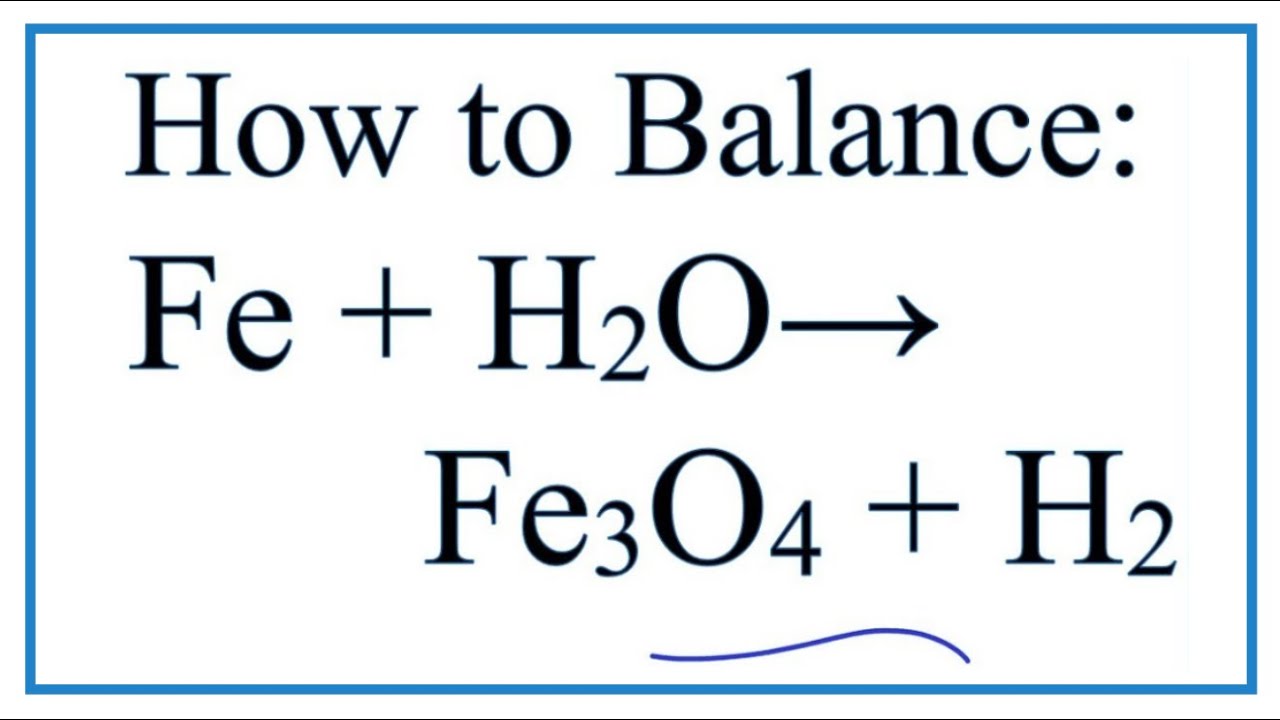Ace Balanced Equation Of Iron And Water

The answer is NO.
Balanced equation of iron and water. Both sides of the equation must have the same quantity of atoms. The balanced equation for this reaction is. Iron reacts with water in the form of steam to form iron oxide along with the release of hydrogen.
An equation for the reaction of. Sodium hydroxide diluted solution. We may NOT add other reactants or products.
What is the balanced equation with state symbols for the reaction of iron II chloride with sodium hydroxide solution. When an iron metal interacts with steam metal oxide and hydrogen gas are the products that are produced. Coke is produced by heating coal in the absence of air.
Because the reaction is carried out in the presence of aqueous acid we can add H as necessary to either side of the equation to balance the charge. Metal steam ----- Metal oxide Hydrogen When red hot iron reacts with steam to form ironIIIII oxide and hydrogen. 2Fes3H2Og Fe2O3s3H2g 2 F e s 3 H 2 O g F e 2 O 3 s 3 H 2 g.
6HCl aq Fe 2 O 3 s 3H 2 O l 2FeCl 3 aq If 12 moles of hydrochloric acid react a The reaction consumes _____ moles of iron III oxide. It displaces hydrogen from watersteam which is evolved or released as a gas. Cells are set up in the metal surface where different areas act as sites of oxidation and reduction.
Iron oxide or rust is the product. 2 The chemical equation for the reaction of calcium with water can be written as. When we balance reaction equations we may ONLY add coefficients to the chemical formulae that are already in the equation.