Ideal Butane And Oxygen Balanced Equation

Write the balanced chemical equation for combustion of butane as shown below.
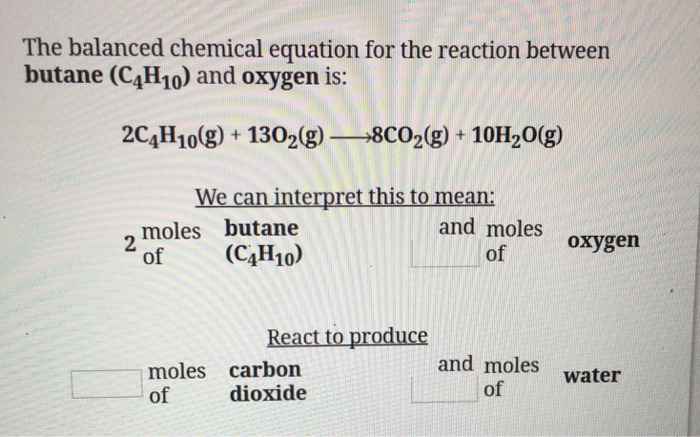
Butane and oxygen balanced equation. 2C4H10g 13O2g 8CO2g 10H2O g. So Butane is the limiting reagent. Butane gas burns in oxygen to form carbondioxide and water.
This is the basic UNBALANCED equation. In order to balance C4H10 O2 CO2 H2O youll need to watch out for two things. We need to think about the number of atoms of each element in the reactants and ensure these are the same in the products.
The combustion of butane is a reaction between butane and oxygen gas that produces carbon dioxide gas and water. Butane is a saturated aliphatic hydrocarbon that contains. From the above equation it is clear that 1 mole of butane reacts to 65 moles of oxygen to give 4 moles of carbon dioxide and 5 moles of water.
The balanced chemical equation for the reaction between butane C4H10 and oxygen is. It will consume first in the balanced equation. Calculate the number of moles of oxygen required to react with 6 moles of butane.
There are two on the left hand side but thirteen on the right hand side so we need to divide thirteen by two to get the scale number which is 65. The equation is thus. C 4 H 10 O 2 ---- CO 2 H 2 O.
An explanation of how to balance the equation C4H10 O2 CO2 H2O and the correct coefficients for each substance in the equationTo balance C4H10 O2. 2C4H10g 13O2g 8CO2g - Answered by a verified Tutor. Butane and oxygen combine in the combustion process and below shows the chemical equation for the combustion of butane.