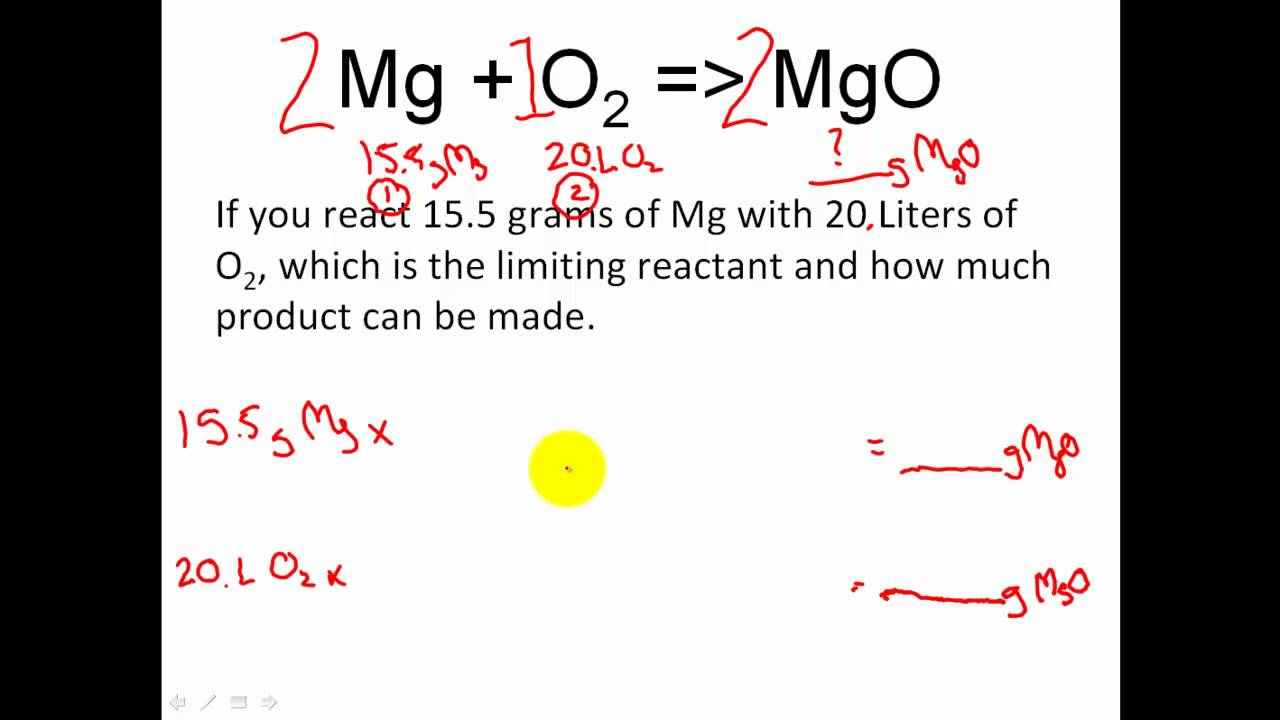Perfect Coefficient Chemical Equation

Balancing Chemical Equations with Interfering Coefficients Vocabulary Coefficient.
Coefficient chemical equation. Here is the example equation again. For example the total number of oxygen atoms in the reacting species 2O 2 is 4. What do coefficients in a chemical equation represent macroscopically.
The total number of atoms of an element present in a species in a balanced chemical equation is equal to the product of the stoichiometric coefficient and the number of atoms of the element in one molecule of the species. It indicates that on the microscopic level 2Hg atoms are required to react with the molecule. A coefficient is a number placed in front of a chemical symbol or formula.
They apply to every part of a product. A coefficient is a whole number multiplier. Get instant feedback extra help and step-by-step explanations.
For example two molecules of hydrogen would be written as 2H 2. Ionic charges are not yet supported and will be ignored. This amount can represent either the relative number of molecules or the relative number of moles described below.
2H2 O2--- 2H2O Note the presence of a two in front of the hydrogen and also the water. Coefficients are used in all chemical equations to show the relative amounts of each substance present. Coefficients number in front of a chemical formula indicate moles of a compound.
Heres something important to remember about coefficients. What do coefficients represent in a chemical equation. When no coefficient is written in front of a species the coefficient is assumed to be 1.