Simple Corrosion Experiment With Nails Report
Corrosion of Iron Objective.
Corrosion experiment with nails report. By roughening the nails surface all these treatmentsbut especially hot-dippingincrease the holding power of the nail. Electrons will move through the wires from the more reactive metal to the less reactive metal. Corrosion is an oxidation-reduction redox reaction in which a free metal is oxidized or corroded by some oxidizing agent.
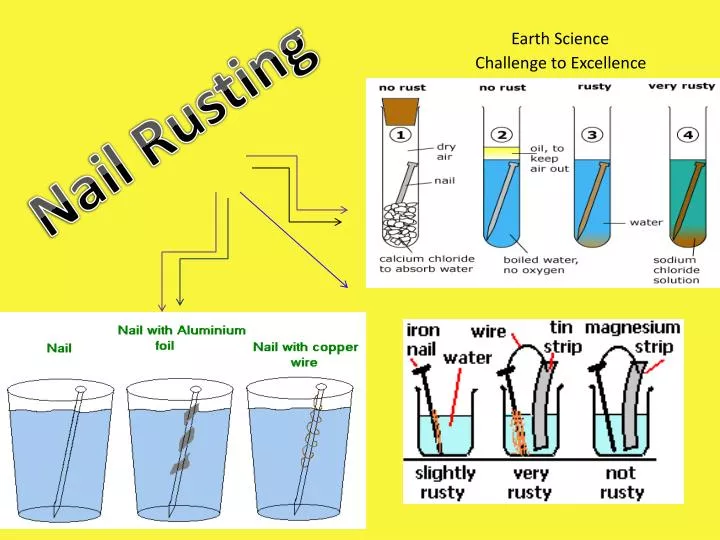
Electrogalvanized nails are plated with zinc and are not as corrosion-resistant as hot-dipped nails. Clean two iron nails with sandpaper or steel wool. The corrosion of iron in an electrolyte solution the effect of a second metal in contact with the iron electrode solution the effect of the relative sizes of the anode and cathode and the potential differences between.
A difficulty experienced during the experiment was that the liquids used reduced significantly in quantity by the end of the second week due to evaporation and had to be. For one finishing nail twist then end of the 7 cm piece of copper wire onto the end of one nail so there are a few coils and the rest of the wire is away from the nail. A third process peens zinc onto the nail.
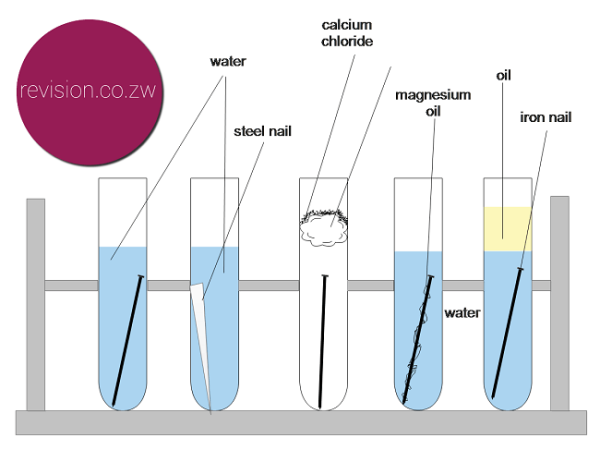
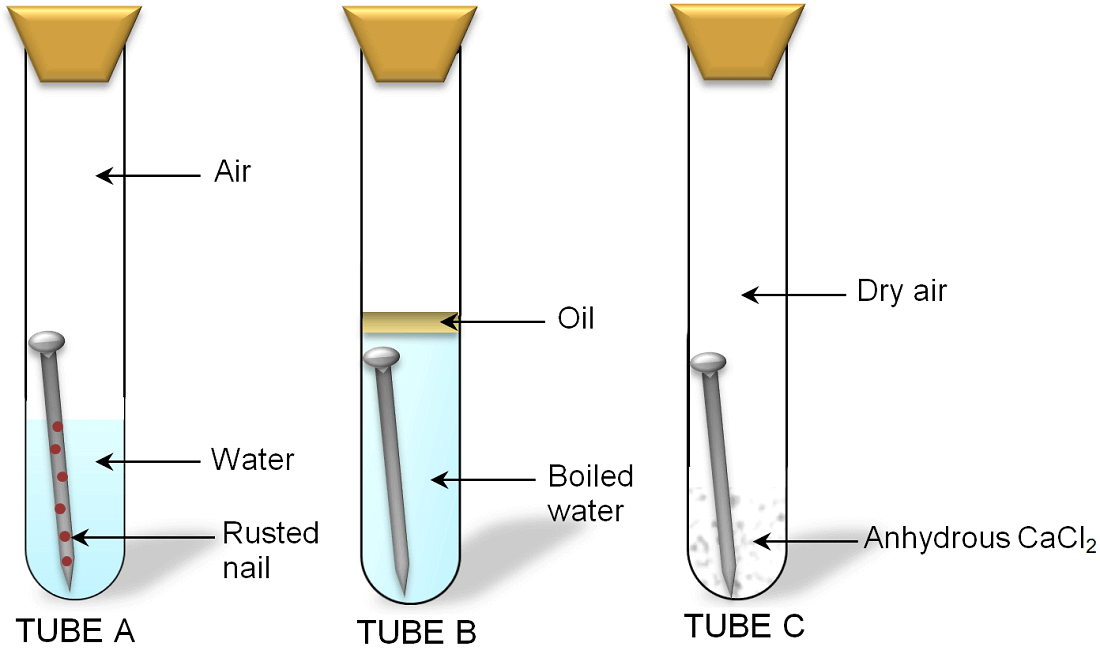
Ready conversion of data to life in years. Explain how atmospheric corrosion occurs and how it results in the eating away of metal. Do not use galvanized nails.
Weigh each nail with an accurate scale at. Take a photo and write down your observations of each nail at the start of the experiment. This paper presents results of simple experiments developed for use in a time-limited course.
This is the case for deformed areas. Effect of relative surface area of the electrodes. Corrosion of Iron Experiment.