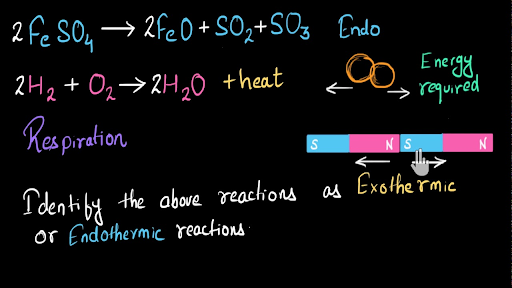Simple Endothermic Reaction Examples Formula
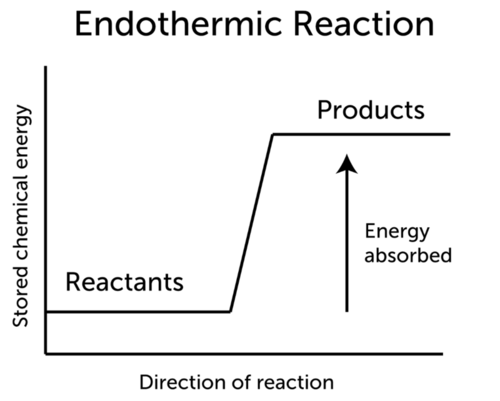
An endothermic reaction uses energy as a reactant.
Endothermic reaction examples formula. In an endothermic reaction heat is used for the reaction to occur. The equation for this reaction is. Common processes and solved examples.
The salt dissociates into ammonium NH 4 and chloride Cl ions. Many processes that happen in daily life give out heat or take in heat for example respiration photosynthesis etc. Heat location is the quickest way to find if a reaction is endothermic or exothermic.
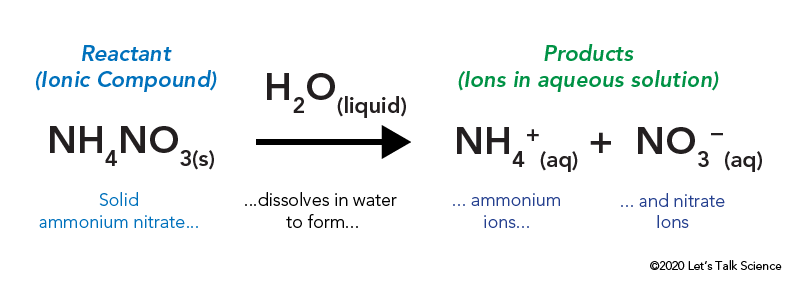
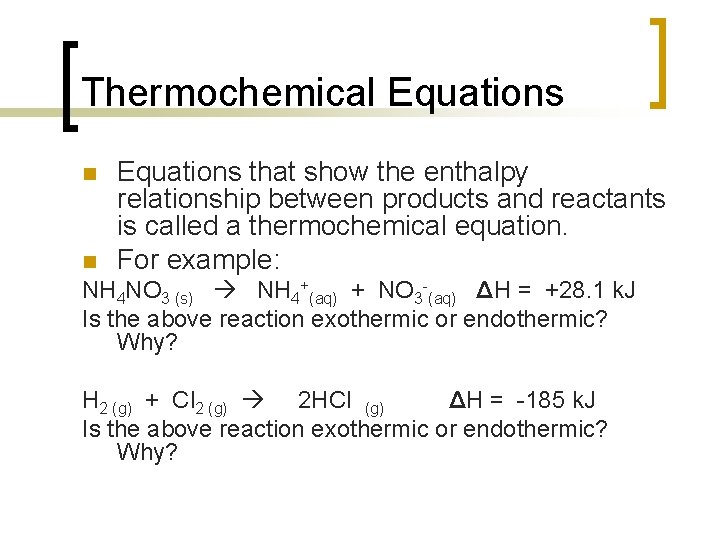
7311 CaCO 3 s CaO s CO 2 g Δ H 1778 kJ. The reaction equationNH4NO3 s water NH4 aq NO3 aqThis is an example of endothermic reaction because the temperature drops because heat energy is taken in by the reaction mixture. Lets categorize them as exothermic or endothermic reactions.
Examples of endothermic reactions. Because the heat is absorbed by the system the 1778 kJ is written as a reactant. When nitrogen and oxygen are heated together to a particular temperature of about 3000 o C nitric oxide gas is.
2 Cooking an egg. This is actually one of the key characteristics of an endothermic reaction. Melting ice is an example of this type of reaction.
Heat energy is absorbed from the pan to cook the egg. These examples could be written as chemical reactions but are more generally considered to be endothermic or heat-absorbing processes. A constant input of energy often in the form of heat is needed to keep an endothermic reaction going.