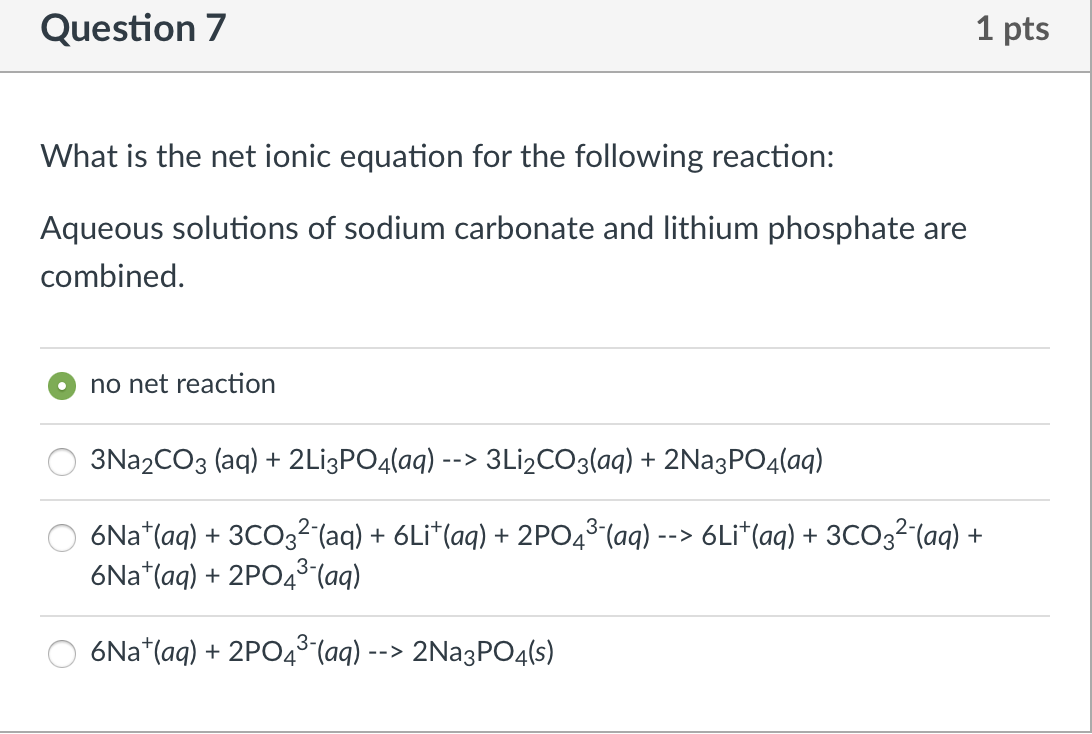
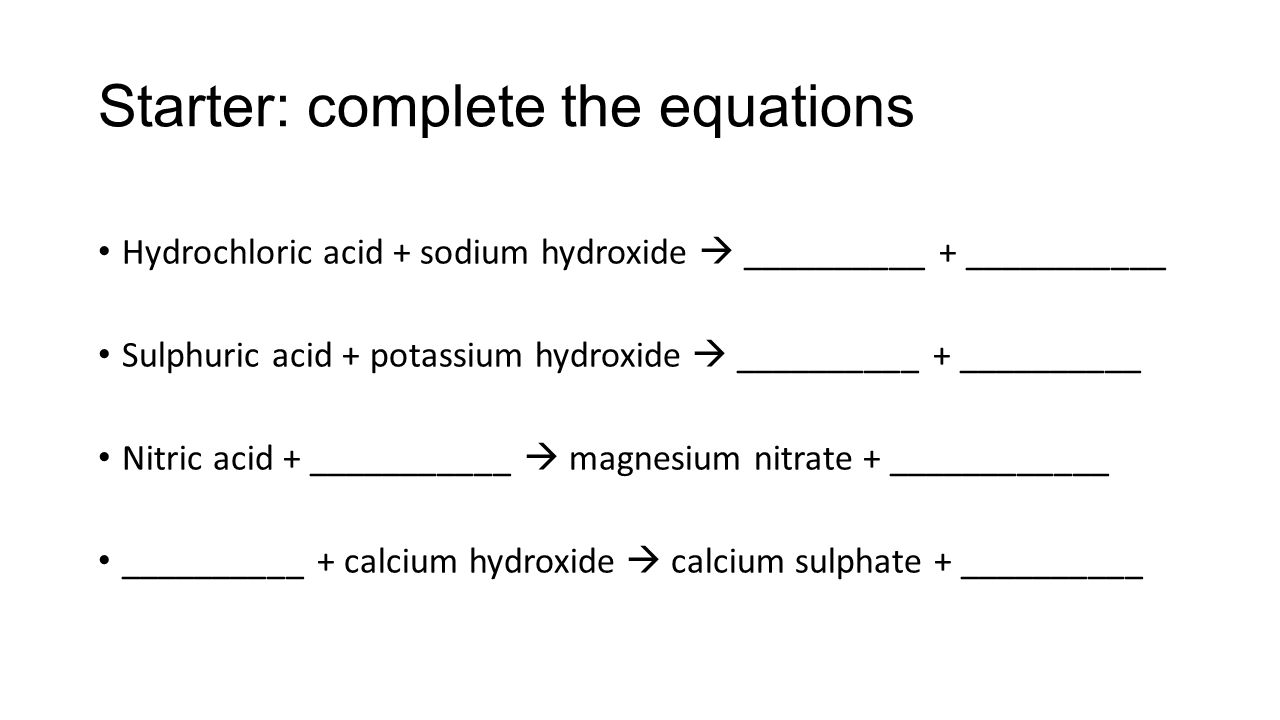
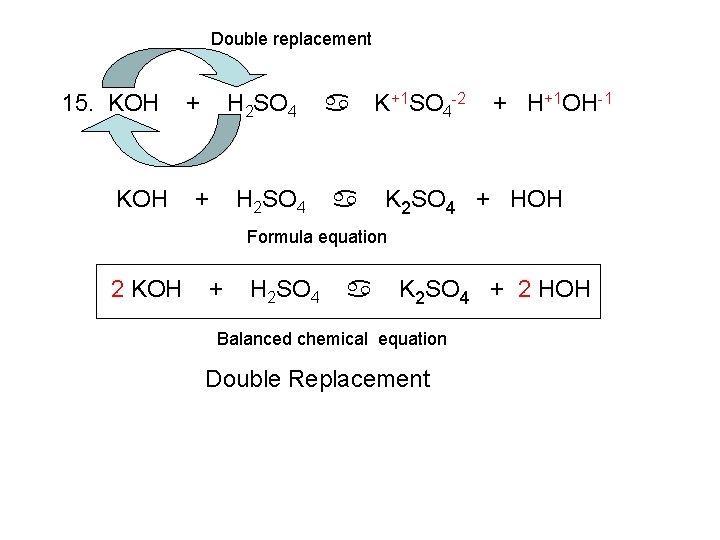
Great Equation For Ammonia And Hydrochloric Acid
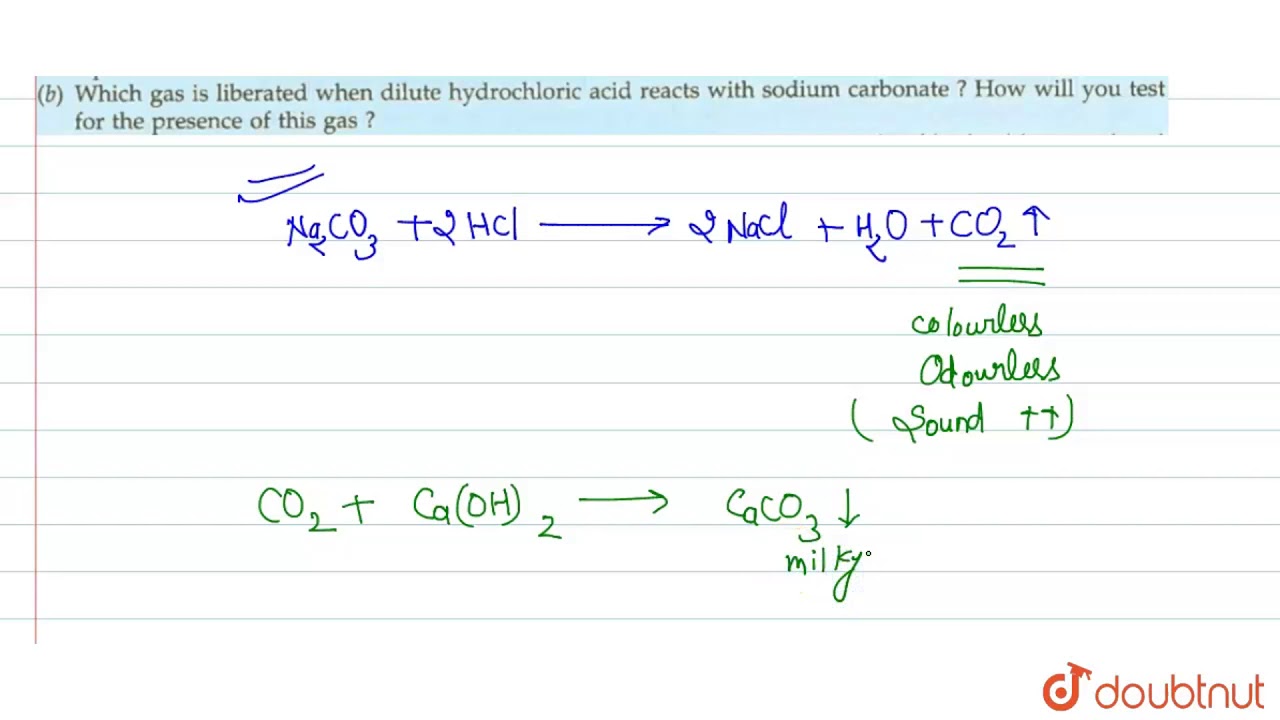
The reaction equation between ammonia NH3 and hydrochloric acid HCl is written as follows.
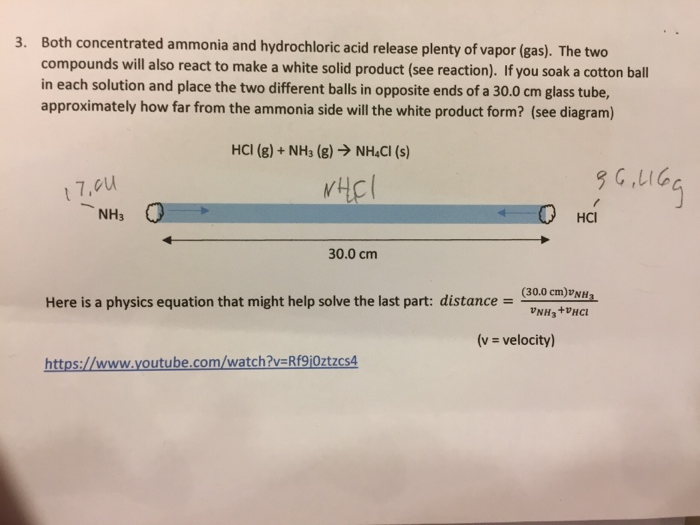
Equation for ammonia and hydrochloric acid. NH 3 aq HClaq NH 4 aq Cl aq Hydrochloric acid and the chlorine ion are one conjugate acid-base pair and the ammonium ion and ammonia are the other. The reaction equation between ammonia NH3 and hydrochloric acid HCl is written as follows. Ammonia is a weak base that reacts with hydrochloric acid forming a compound called ammonium chloride.
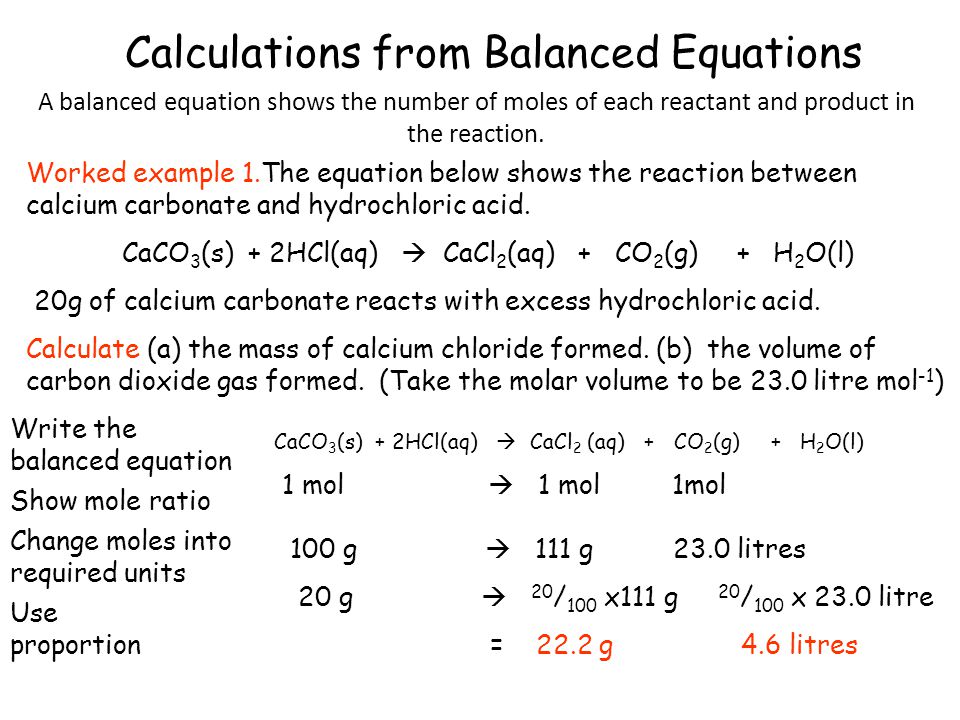
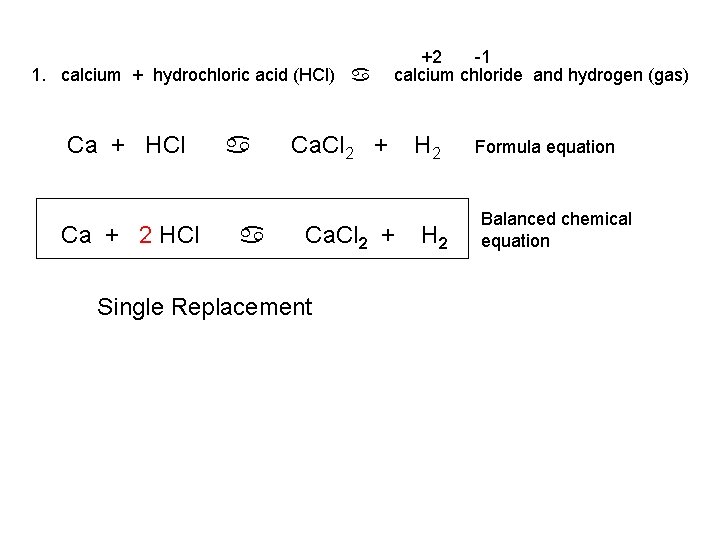
To write the products we combine the anion of the acid with the cation of the base and write the correct formula following the principle of electroneutrality. Additionally is HCl a strong acid. The reaction of hydrochloric acid HCl with ammonia NH3 is described by the equation.
Explaining the direct combination reaction between ammonia solution and concentrated hydrochloric acidthe product of this reaction is white fumes of ammoniu. The reaction can be balanced as. Write a net ionic equation for the reaction that occurs when an aqueous solution of hypochlorous acid and potassium hydroxide are combined.
The reaction equation between ammonia NH3 and hydrochloric acid HCl is written as follows. The chemistry of the titration of the weak base ammonia with the strong acid hydrogen chloride is captured by the neutralization reaction NH 3 aq HClaq NH 4 Claq H 2 Ol. When ammonium chloride decomposes ammonia and hydrochloric acid are formed.
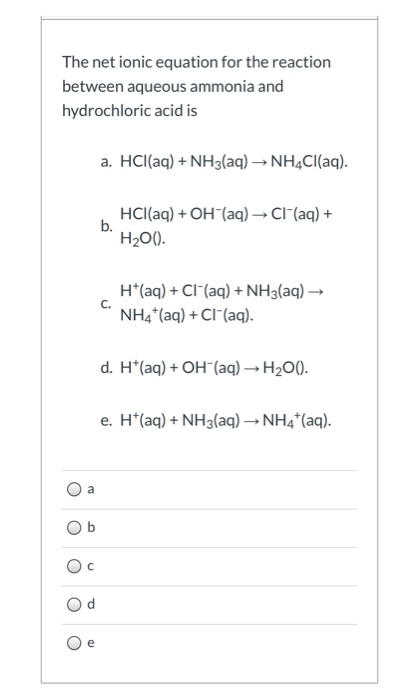
Haq OHaq H2Ol. Ammonia is a weak base that reacts with hydrochloric acid forming a compound called ammonium chloride. When carbonic acid decomposes water and.
HClaq H 2 O l H 3 O aq Cl aq Using the Brønsted-Lowry theory the reaction of ammonia and hydrochloric acid in water is represented by the following equation. The reaction equation between ammonia NH3 and hydrochloric acid HCl is written as follows. In this case the coordination number of the copper changes from six to four.