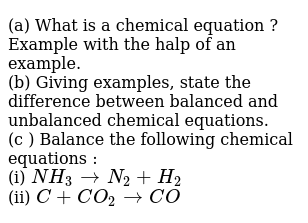Glory Skeletal Chemical Equation Definition
Balanced Chemical Equation Definition Examples Video Lesson.
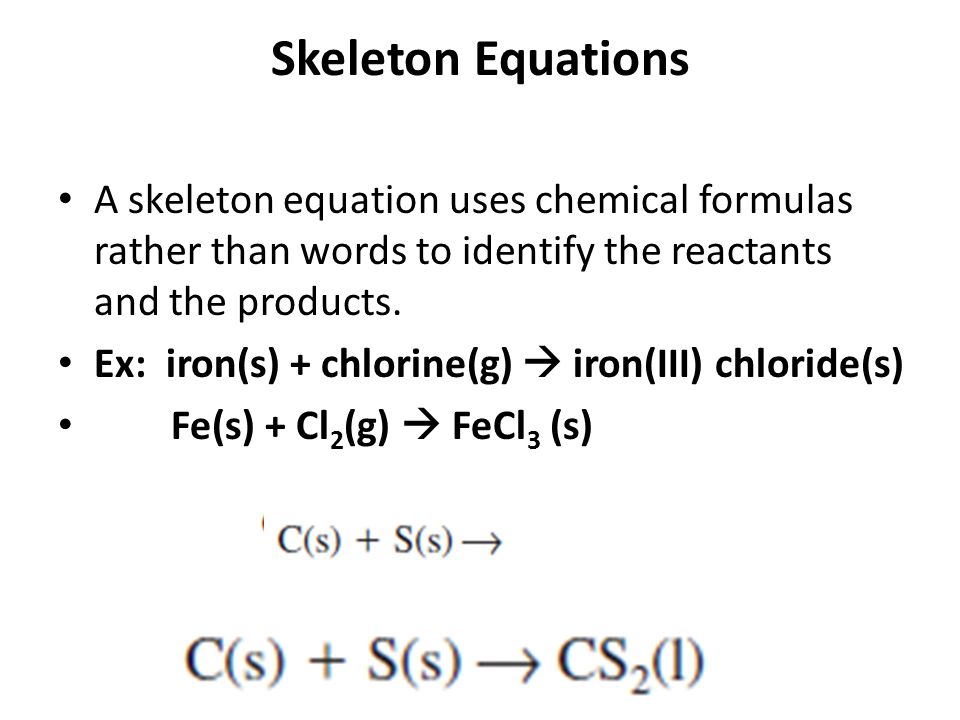
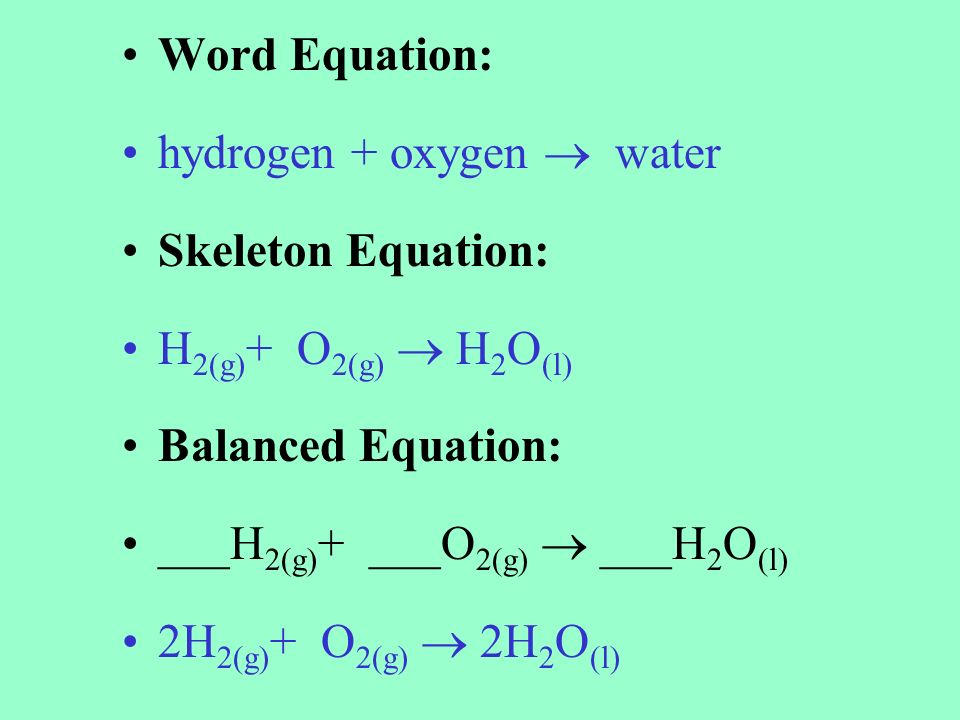
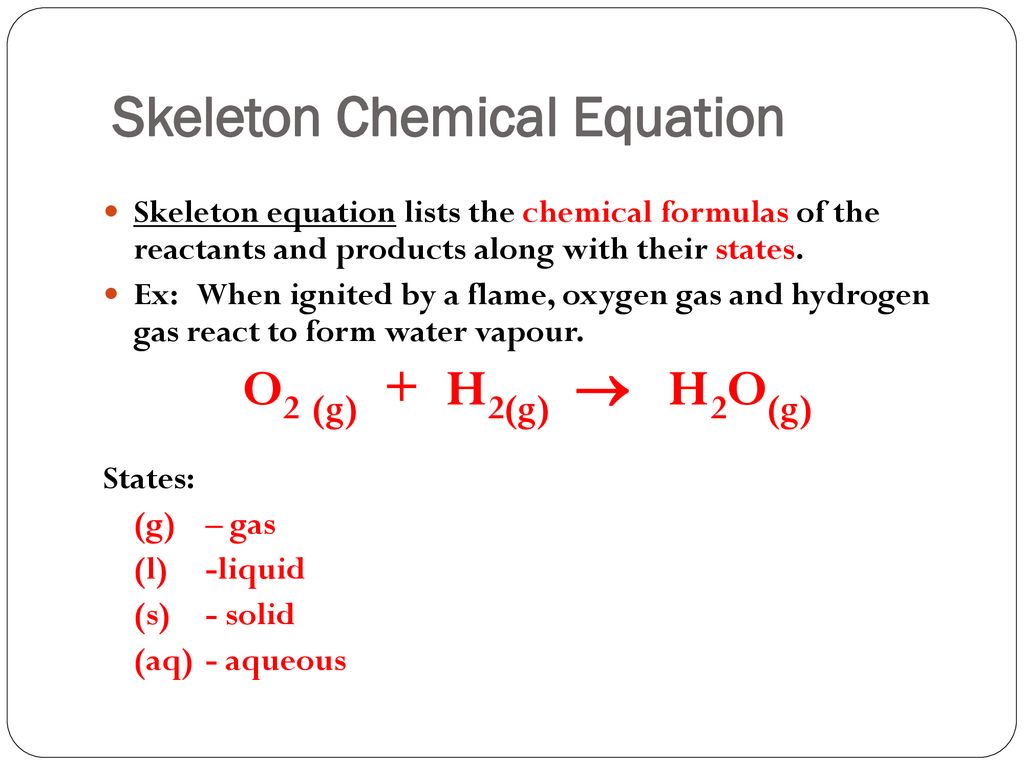
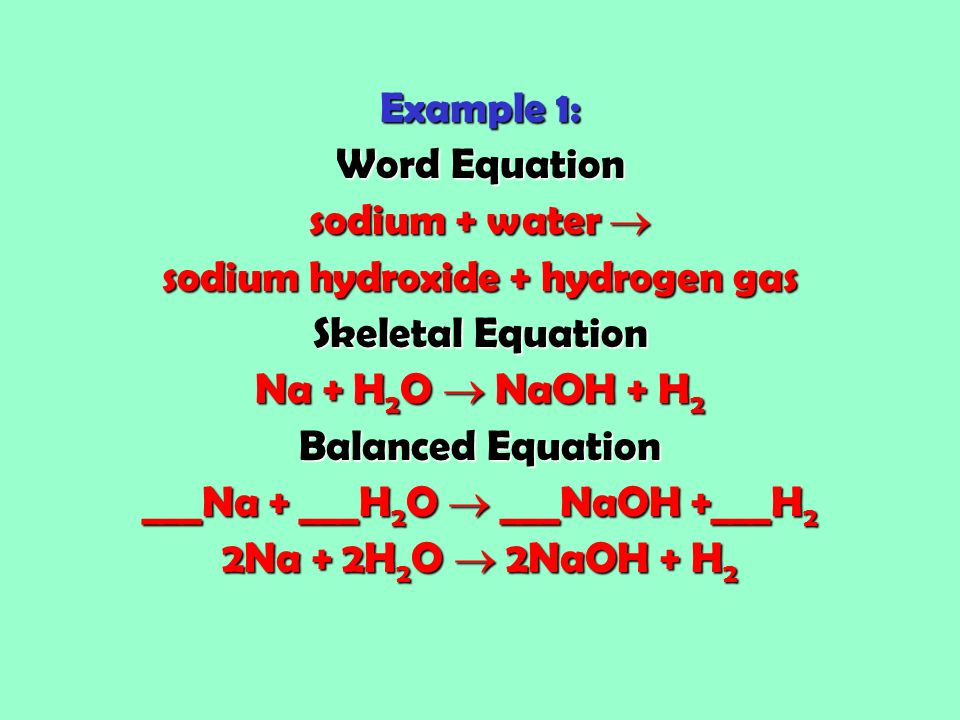
Skeletal chemical equation definition. A skeleton equation by definition is a way of using formulas to indicate the chemicals that are a part of the chemical reactionIn essence it is identical to a word equation except that the names of the reactants and the products are substituted by their chemical symbols. The next step is Balancing Chemical EquationsTable of C. Friday September 21 2018.
How To Write Skeleton Equations Youtube. The key difference between balanced equation and skeleton equation is that balanced equation gives the actual number of molecules of each reactant and product involved in the chemical reaction whereas skeleton equation gives only the reactants of the. Skeletal equations are equations which show the reactants and the products so formed without balancing them.
How is a skeleton equation different from a balanced equation. Definition of of balanced chemical reaction In a chemical reaction total mass of the all reactants are equal to total mass of the all the products. The chemical formula for the reactant 1 is C9H9O2Br C 9 H 9 O 2 B r.
A skeleton equation by definition is a way of using formulas to indicate the chemicals that are a part of the chemical reaction. Later it has to be balanced by appropriate number of molecules. Definition of Word-equation 2.
A skeletal or skeleton chemical equation is an unbalanced equation. In essence it is identical to a word equation except that the names of the reactants and the products are substituted by their chemical symbols. H 2 O 2 H 2 O.
Definition of Balanced Chemical. 49 Balancing Chemical Equations Worksheets With Answers. Why we need to balance a skeletal chemical equation.