Glory What Is A Balanced Chemical Equation Definition

A chemical equation is the symbolic representation of a chemical reaction in the form of symbols and formulae wherein the reactant entities are given on the left-hand side and the product entities on the right-hand side.
What is a balanced chemical equation definition. A balanced chemical equation occurs when the number of the. As a result the chemical equation that shows the chemical reaction needs to be balanced. The first chemical equation was diagrammed by Jean Beguin in 1615.
Use uppercase for the first character in the element and lowercase for the second character. They also make use of symbols to represent factors such as the direction of the reaction and the physical states of the reacting entities. Fe Au Co Br C O N F.
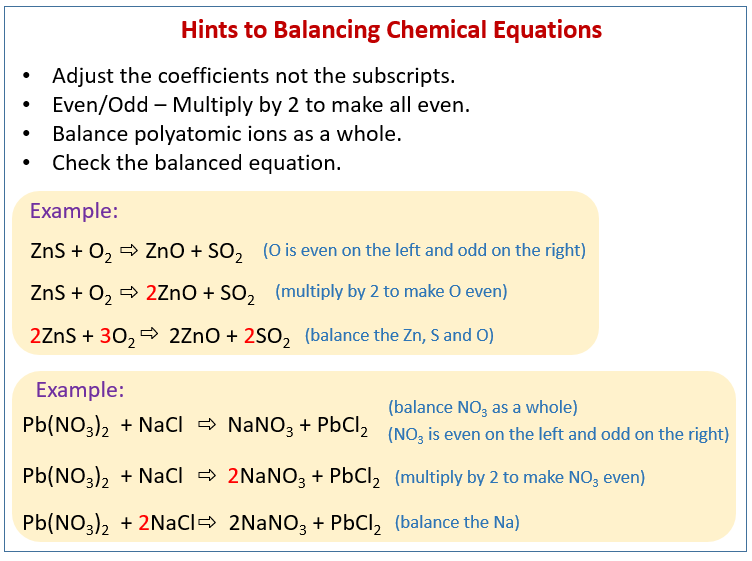
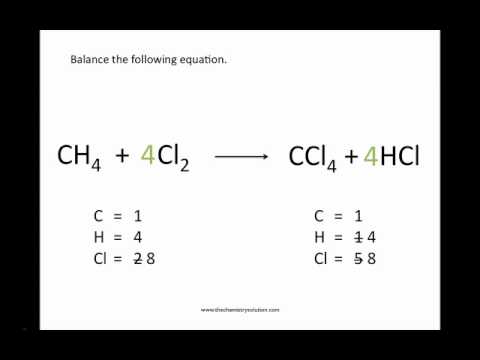
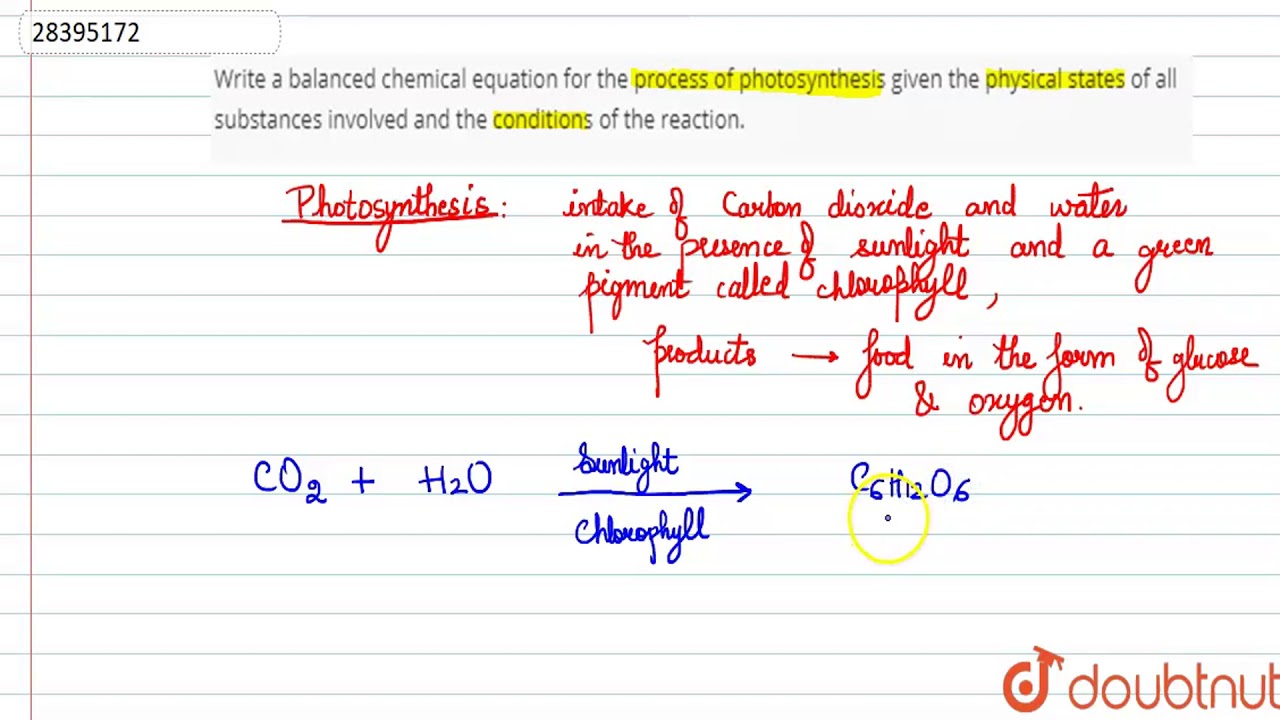
A balanced chemical equation occurs when the number of the atoms involved in the reactants side is equal to the number of atoms in the products side. As a result the chemical equation that shows the chemical reaction needs to be balanced. It is a fully detailed equation which gives the ratios between reactants and products.
A balanced equation is an equation for a chemical reaction in which the number of atoms for each element in the reaction and the total charge is the same for both the reactants and the products. Glycolysis is a series of biochemical reactions that break down a glucose molecule into two molecules of pyruvic acid. Coefficients number in front of a chemical formula indicate moles of a compound.
What are the products of combustion of benzene. What is the definition of balanced chemical equation. The reactant chemical s are given on the left-hand side and the product chemical s on the right-hand side.
The chemical equation written by balancing total number of atoms of each element in reactants and products It is more informative than the unbalanced chemical equation. A balanced equation is an equation for a chemical reaction in which the number of atoms for each element in the reaction and the total charge is the same for both the reactants and the products. Law of conservation of mass governs the balancing of a chemical equation.