Out Of This World Ammonia And Hcl Reaction Equation

How do you write an equation to represent Ammonia reacts with hydrochloric acid to form ammonium chloride.
Ammonia and hcl reaction equation. HCl aq H 2 O l H 3 O aq Cl aq Using the Brønsted-Lowry theory the reaction of ammonia and hydrochloric acid in water is represented by the following equation. You could write this equation in two ways. The reaction equation between ammonia NH3 and hydrochloric acid HCl is written as follows.
Chemistry Chemical Reactions Chemical Reactions and Equations. In solution we write it as H3Oaq Cl -aq. The reaction equation between ammonia NH3 and hydrochloric acid HCl is written as follows.
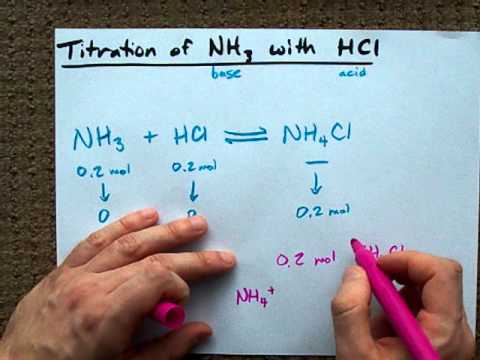
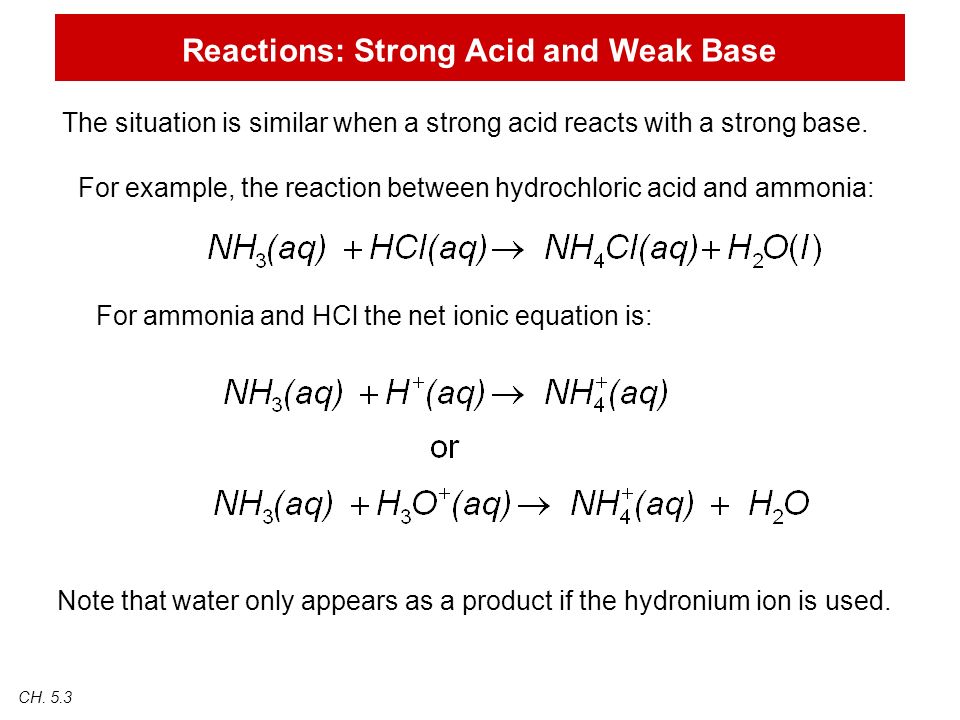
NH 3 aq HCl aq NH 4 aq Cl aq Hydrochloric acid and the chlorine ion are one conjugate acid-base pair and the ammonium ion and ammonia are the other. Reaction of ammonia with oxygen with catalyst and steps of balancing the chemical equation Uses of this reaction in industrial scale productions Ammonia and oxygen without catalyst NH 3 O 2 N 2 H 2 O With supply of heat ammonia reacts with oxygen and produce nitrogen gas and water as products. NH3aq HClaq NH4Claq NH4OHaq HClaq NH4Claq H2Ol.
If exposed wash off immediately under cold running water. HCl aq NaOH aq --- NaCl aq H 2 O l So the molecular form of the equation is shown above. By its chemical nature the nitrogen in ammonia prefers to be attached to four hydrogens rather than the mere three it has so it steals the hydrogen from hydrogen chloride.
R Br excess NH 3 R NH2 Access. How much hydrochloric acid must be added to react completely. One may also ask what is the chemical reaction for ammonia.
For example dilute ethanoic acid reacts with magnesium. In order to write the chemical equation you need to know the correct chemical formulas for. Reactions of carboxylic acids with metals.