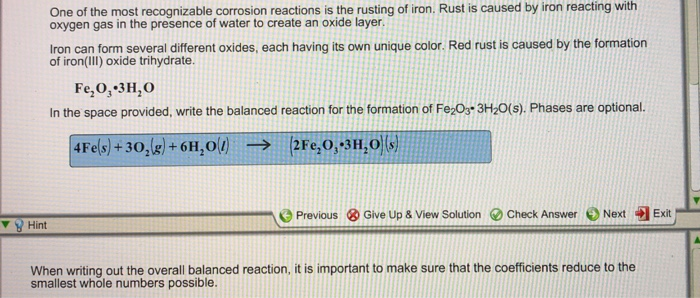
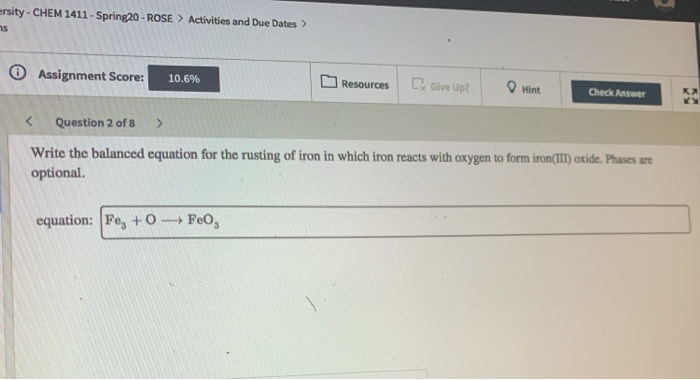
Impressive Balanced Equation For The Rusting Of Iron

The formation of rust requires iron water and oxygen.
Balanced equation for the rusting of iron. Rusting is the corrosion of iron. The surface of iron at the middle of the water droplet. Beside above what is the balanced equation for Fe2O3 co yields Fe co2.
Solution for write the balanced equation for the rusting of iron in which iron reacts with oxygen to form ironiii oxide. The two half - cells are. The formula is approximately Fe 2 O 3 3 2 H 2 O although the exact amount of water is variable.
Iron water oxygen hydrated iron III oxide Iron and. Does iron rust underwater. Fe - Fe 2 2e -.
The oxidation reaction of iron and oxygen to form the substance that is commonly called rust occurs according to this equation. Oxidation of Solid Iron Its common knowledge that rust occurs when you leave water on a metal implement or you leave it. What is rusting of iron Class 8.
Here is the word equation for the reaction. Note that this is about halfway between iron III hydroxide Fe OH 3 or ½ Fe 2 O 3 3H 2 O and anhydrous Fe 2 O 3. Rusting is an electrochemical process.
Rusting is a redox reaction whereby oxygen acts as the oxidising agent and iron acts as the reducing agent. The chemical formula for rust is Fe 2 O 3nH 2 O The overall chemical equation for the formation of rust is Iron water oxygen rust 4 Fe s 6 H 2 O l 3 O 2 g 4 Fe OH 3 s. The rusting of iron usually is considered to be a destructive change and considerable time and money are expended to prevent it.