Recommendation Ammonia React With Carbon Dioxide To Form

6372g of ammonia are reacted with 7873 g of carbon dioxide.
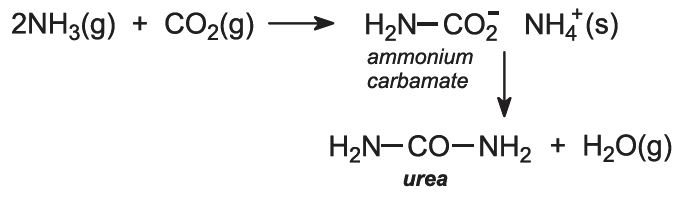
Ammonia react with carbon dioxide to form. The separation of carbon dioxide from the aqueous ammonia. When combining ammonia gas with carbon dioxide also a gas we get Urea of which the chemical formula is CO NH22 and the chemical reaction will be. Heres a video of the reaction.
A reboiler is used in the stripper to provide the energy needed for the salts formed to dissociate and for the carbon dioxide to escape to the gas phase. Rapid decomposition of carbamic acid occurs without enzyme catalysis to form ammonia and carbon dioxide. 2NH3 CO2 - NH22CO H2O So essentially the same as the formula in Abel Palmers formula.
Check Answer and Solution for above question f. Determine the mass of oxygen reacted. H2O NH2CONH2 urea.
O2 g 2CO g 2CO2 g If a sample of 700 g of carbon oxide was reacted completely with 320 g of oxygen how many moles of carbon dioxide would be produced. The pressure and temperature is maintained at 14 Mpa and 170-190C for the first reaction to occur. NH3 aq CO2 aq H2O l NH4HCO3 aq Suppose 215 g NH3 552 g CO2 and 100 mol H2O are reacted.
Urea synthesis Urea is made from ammonia and carbon dioxide. Urea NH22CO is prepared by reacting ammonia with carbon dioxide. Reversible reaction of ammonia with carbon dioxide in multiple steps to ultimately form ureaThis video is about.
NH2COONH4 ammonium carbamate NH2COONH4. 20When black gunpowder explodes potassium nitrate carbon and sulfur react with each other to form nitrogen carbon dioxide and potassium sulfide. 3 - the decomposition of ammonium carbonate - total reaction.