Unique An Iron Nail Rusts Evidence Of Chemical Change
When substances made of iron are exposed to oxygen and moisture water rusting takes place.
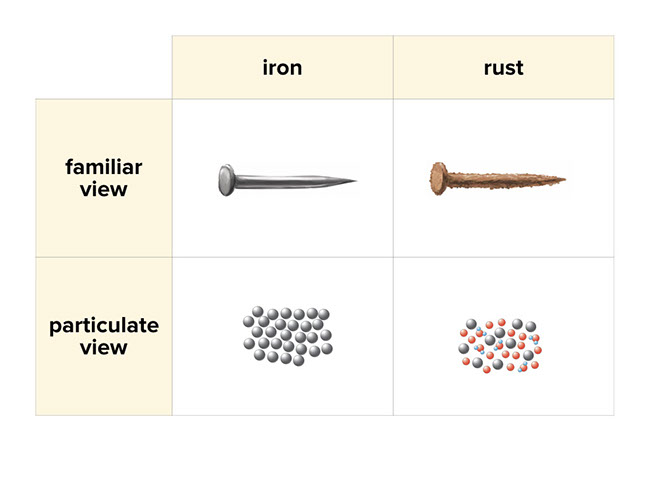
An iron nail rusts evidence of chemical change. Rusting is an example of a chemical change. It results in the formation of Iron Oxide which is an entirely new substance. Rust requires three chemicals in order to form.
In these circumstances we break iron-iron bonds and oxygen-oxygen bonds and form F eIIO and F eIIIO bonds. The rust is produced over time naturally. The following can indicate that a chemical change.
Rusting of iron refers to the formation of rust a mixture of iron oxides on the surface of iron objects or structures. Write down the evidences which prove that the chemical change occurs in each process. A silver spoon tamishes.
When an iron nail rusts its mass. Is the formation of rust on an iron nail a chemical change or a physical change. A silver spoon tamishes.
It is a chemical change because you cannot undo rusting and a new substance- the rust- is formed PrincessAnna00 PrincessAnna00 04032016 Physics Middle School Is the formation of rust on an iron nail a chemical change or a physical change. This is due to the formation of iron oxide which is the reaction between iron and oxygen. Rusting is a chemical change.
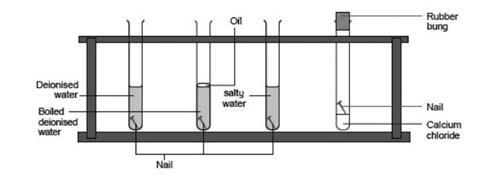
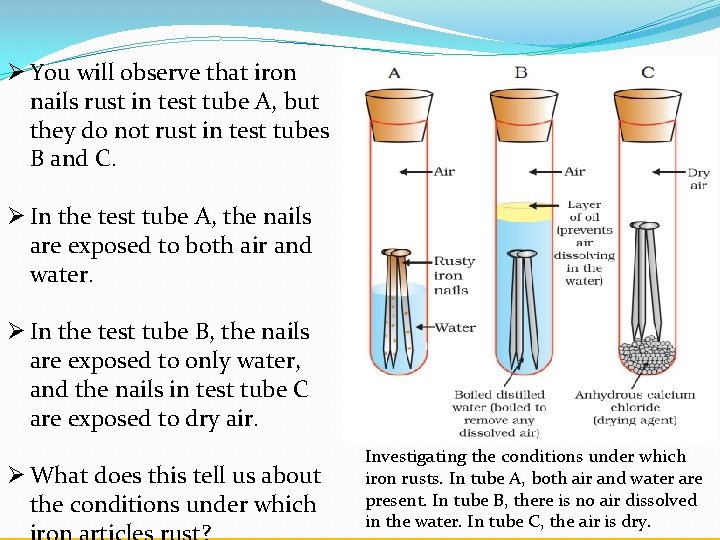
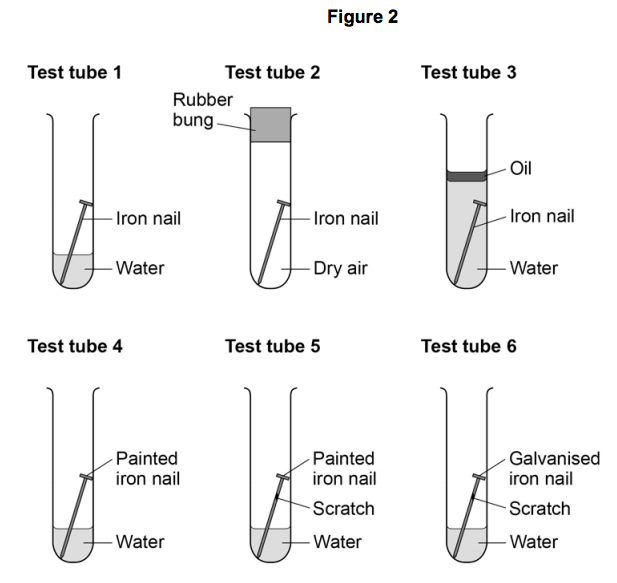
Although about 21 of air consists of oxygen 1 rusting doesnt occur in dry air. Rust is clearly a substance that is different from iron. Iron Oxygen from environment Water Humidity Iron Oxide Rust.