Marvelous Ammonia + Sulfuric Acid Equation
The reaction of ammonia with sulfuric acid is described by the following chemical reaction equation.
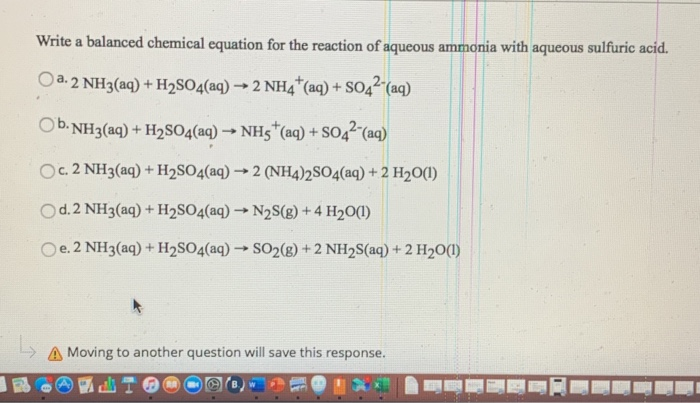
Ammonia + sulfuric acid equation. 2NH3 H2SO4 NH4 2SO4. By signing up youll get. Ammonia and sulfuric acid react to form ammonium sulfate.
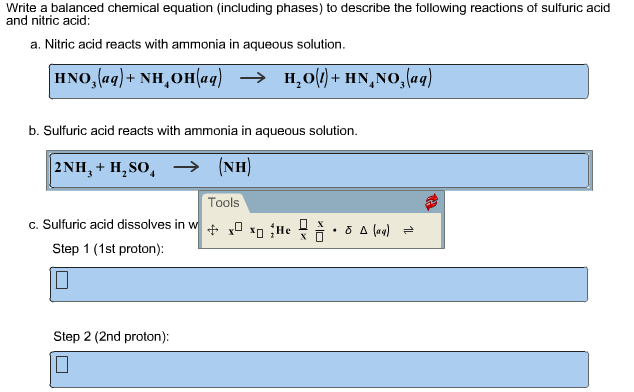
The balanced equation for the reaction of aqueous sulfuric acid with aqueous ammonia is. H 3 NSO 3 H 2 O H 2 SO 4 NH 3. Ammonia sulfuric acid ammonium sulfate ammonium hydroxide sulfuric acid ammonium sulfate water Sulfuric acid is also used in the production of detergents and paints.
Ammonia NH3 is a weak base and sulfuric acid H2SO4 is a strong acid so their reaction will be an acid base reaction. NH3 H2SO4 NH42SO4 Chemical reaction and equation Ammonia react with sulfuric acid 2NH 3 H 2 SO 4 NH 4 2 SO 4 Check the balance. Sulfuric acid is used in the manufacture of paints detergents and fertilisers.
To obtain one mole of ammonium sulfate two moles of ammonia and one mole of sulfuric acid are required. The p-Block Elements. As a result of the reaction of potassium permanganate KMnO 4 sulfuric acid H 2 SO 4 and ammonia.
2NH3aq H2SO4 aq -- NH42SO4aq a. Chemical equation of reaction of 6KMnO4 9H2SO4 5NH3 5HNO2 3K2SO4 6MnSO4 14H2O. Sulfuric acid ammonium hydroxide water ammonium sulphate H 2 SO 4 2NH 4 OH 2H 2 O NH 4 2 SO 4 Use previously standardised 005M sulfuric acid.
Vocabulary of Myasthenia Gravis Muscular Dystrophy. However the sulfamic acid reverts to sulfuric acid in place of the CO 2 that urea would release according to the following reaction. The product of all acid base reactions is a.