Spectacular Chemical Equation Of Ammonia And Hydrochloric Acid

134 Hydrochloric acid has an irritating pungent odor with an odor threshold of about 7 mgm 3.
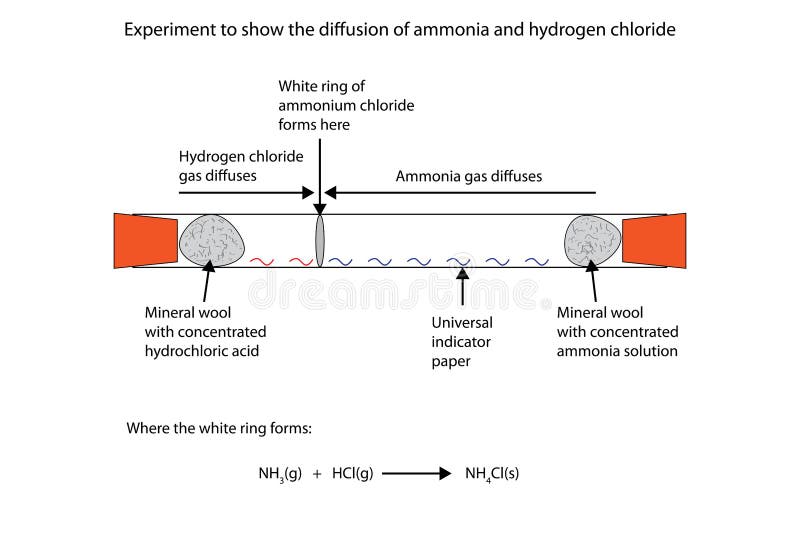
Chemical equation of ammonia and hydrochloric acid. The reaction equation between ammonia NH3 and hydrochloric acid HCl is written as follows. B Write a balanced chemical equation for the reaction and include the heat value in the. The reaction equation between ammonia NH3 and hydrochloric acid HCl is written as follows.
How to tell if a chemical reaction has occurred. Based on signs of new substances formed with properties different from reactants to identify whether a chemical. For the reaction between ammonia and hydrochloric acid to produce ammonium chloride.
HCl aq H 2 O l H 3 O aq Cl aq Using the Brønsted-Lowry theory the reaction of ammonia and hydrochloric acid in water is represented by the following equation. 13 Hydrochloric acid occurs as a colorless nonflammable aqueous solution or gas. Call Us 247 905338587624.
Ammonia is a weak base that reacts with hydrochloric acid forming a compound called ammonium chloride. The reaction for zinc and hydrochloric acid would be zinc plus hydrochloric acid produces hydrogen plus zinc chloride. Male rats gavaged with 1000 umol 15N-ammonium chloride each day for 5 days excreted low but significant amounts of excess 15N-NO3- in urine on the 5 days of treatment on the 5 subsequent daysAn in vitro chemical model system was used to demonstrate that oxidation of ammonia to NO3- by the hydroxyl radical at physiological pH is chemically feasible.
NH 3 aq HCl aq NH 4 aq Cl aq Hydrochloric acid and the chlorine ion are one conjugate acid-base pair and the ammonium ion and ammonia are the. Ammonia is a weak base that reacts with hydrochloric acid forming a compound called ammonium chloride. Basic hydrolysis gives a salt of the carboxylic acid and ammonia or an amine.
A word equation represent the reactions between metals and acids. Chemical equation of reaction of NH3 HCl NH2Cl 2H. The reaction equation between ammonia NH3 and hydrochloric acid HCl is written as follows.