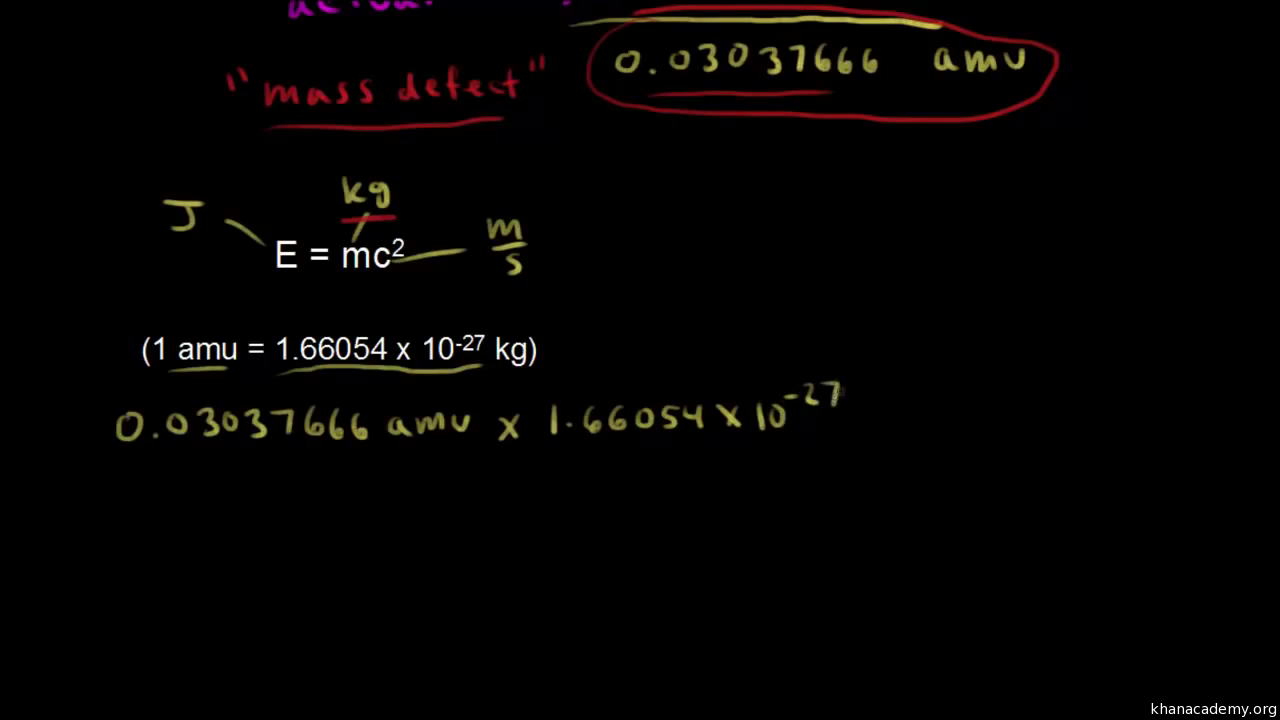Neat Formula For Mass Defect

This approach is an extension of the concepts of Kendrick mass transformation widely used for identification of homologous compounds differing only by a number of base units eg CH2 H2 O CH.
Formula for mass defect. Find the mass defect of a copper-63 nucleus if the actual mass of a copper-63 nucleus is 6291367 amu. Removing energy reduces mass. According to the formula when energy increases mass and inertia increase.
Higher-order mass defect analysis is introduced as a unique formula assignment and visualization method for the analysis of complex mass spectra. Mass defect also called mass deficit is the difference between the mass of an object and the sum of the masses of its constituent particles. The course teaches Advanced Nuclear Theory.
During a nuclear fusion reaction mass defect is converse to energy and can be calculated by the following formula where Z is the proton number A is the atomic mass number mp is the mass of proton m n is the mass of neutron mN is the mass of atomic nucleus. 1 Z m p A Z m n m N Δ m. Does an electron have any significant mass.
The difference between the mass of a nucleus and the sum of the masses of the nucleons of which it is composed is called the mass defect. The actual mass of the nucleus the. Mass defect is expressed in unit amu or Kg.
Show that an alternative formula for the mass defect is deltaZ M_HA-Z m_n-M_text atom where M_H is the mass of a hydrogen atom and m_n is th Announcing Numerades 26M Series A led by IDG Capital. Add up the masses of each proton and of each neutron that make up the nucleus subtract the actual mass of the nucleus from the combined mass of the components to obtain the mass defect. The mass defect 𝚫M can be calculated by subtracting the original atomic mass M A from the sum of the mass of protons m p 100728 amu and neutrons m n 100867 amu present in the nucleus.
Whereas electron is present outside the nucleus and do not contribute for the atomic mass. Proton and neutron are present inside nucleus and the sum of these two is equal to atomic massmass number of nucleus or element. The binding energy of a system can appear as extra mass which accounts for this difference.