Casual Incomplete Combustion General Equation

Recognizing the characteristics and balancing incomplete combustion reactions.
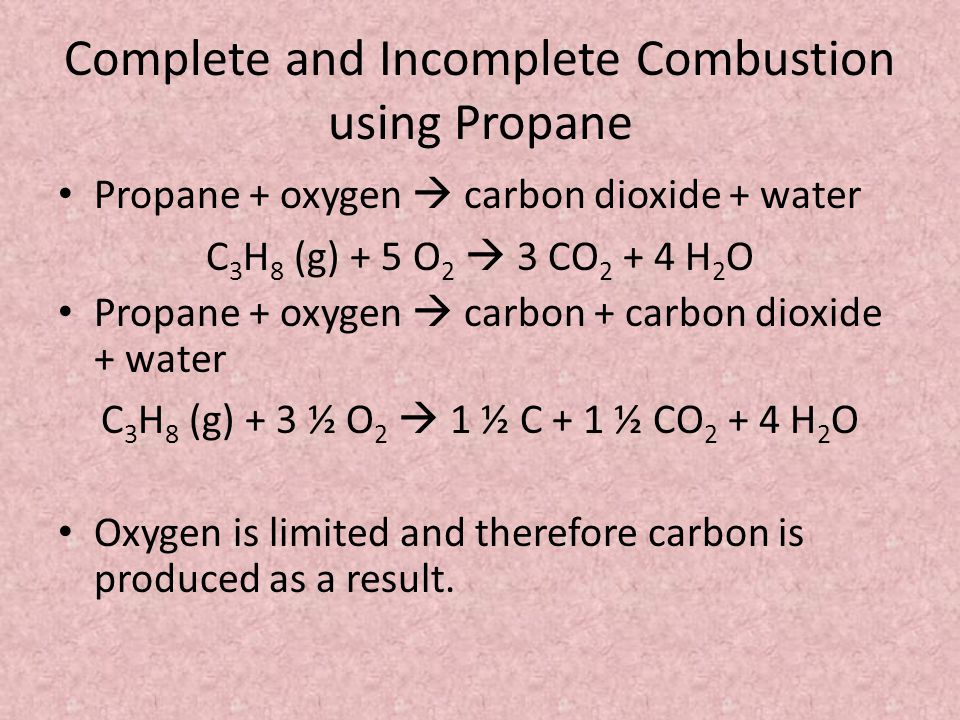
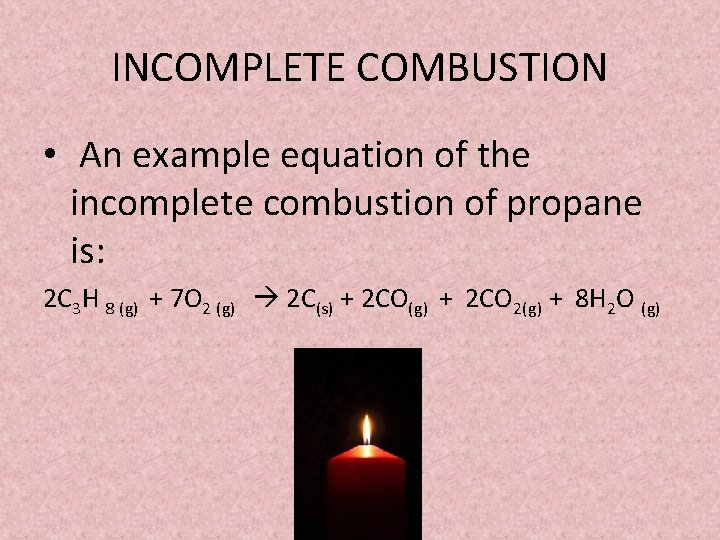
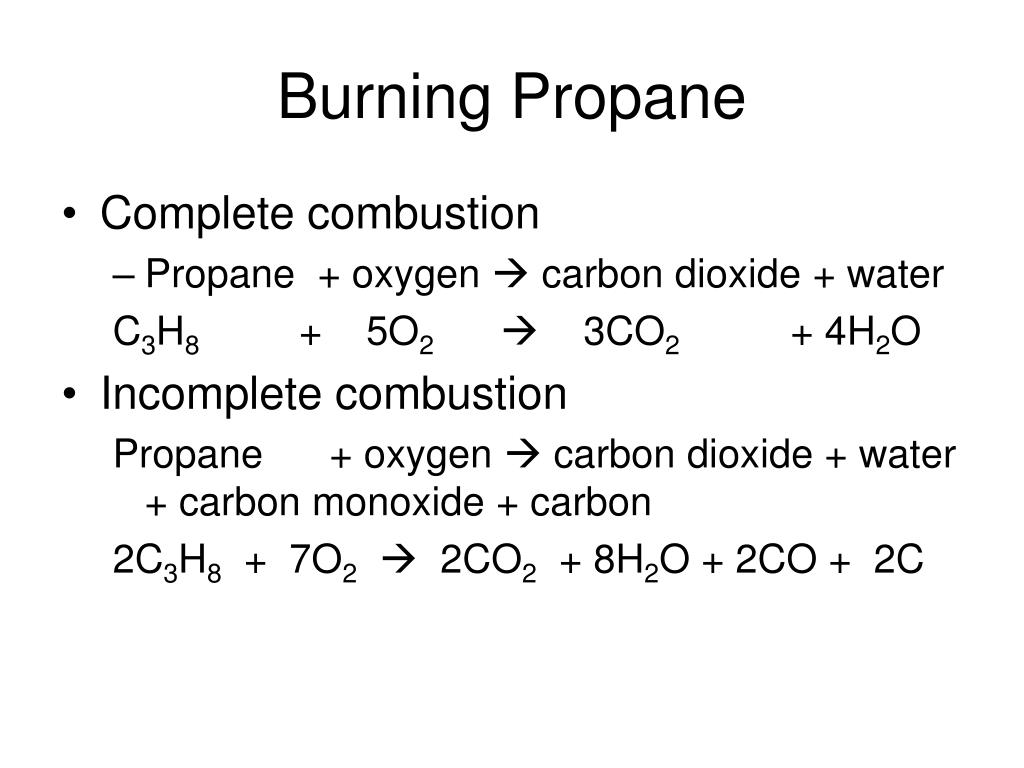
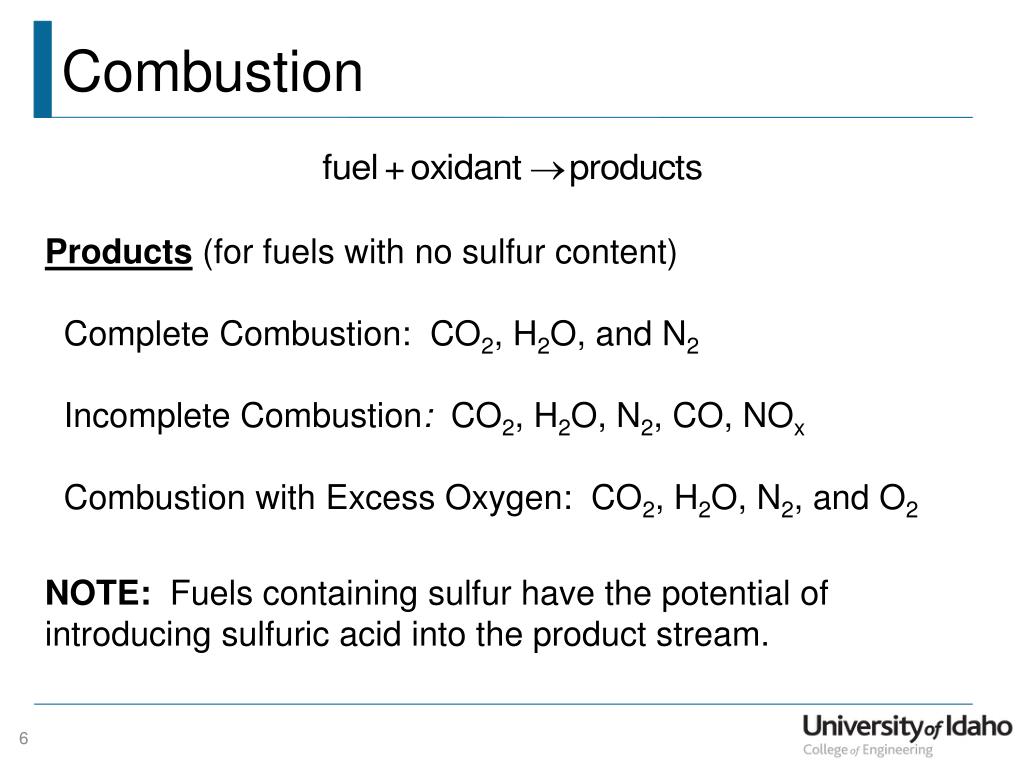
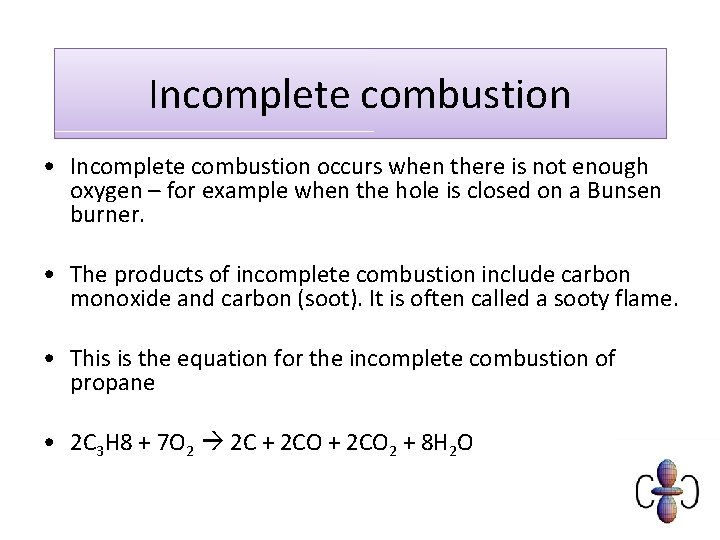
Incomplete combustion general equation. It usually occurs when a hydrocarbon reacts with oxygen to produce carbon dioxide and water. 2C3H 8 g 7O2 g 2C s 2CO g 2CO2 g 8H2O g 8. In general for incomplete.
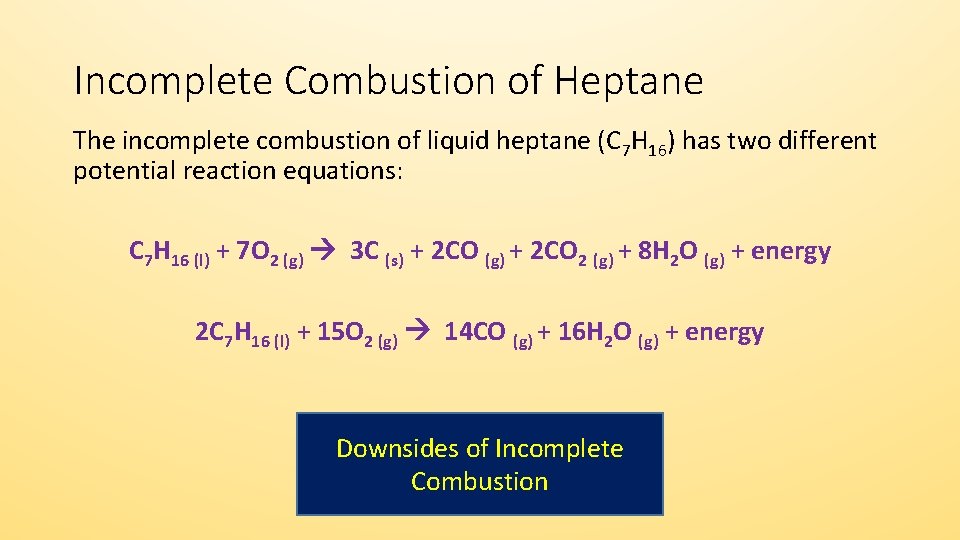
A Incomplete Combustion Equation - Multiple carbon products Hydrocarbon Oxygen Carbon Carbon monoxide water When writing equations with incomplete combustion it is advisable to include only one carbon product otherwise there will be multiple solutions to the equation. For example sometimes it yields only carbon monoxide or soot. It is possible to give an equation for complete combustion but the equation depends on what is being combusted.
Incomplete combustion is also a reaction between oxygen and fuel but the products are carbon monoxide water and carbon. Incomplete combustion occurs when the supply of air or oxygen is poor. Incomplete combustion where there is not enough oxygen present can lead to the formation of carbon or carbon monoxide.
For example CH4 C2H4 H2S are all gases which can burn but will have different equations for complete co. As a simple way of thinking about it the hydrogen in the hydrocarbon gets the first chance at the oxygen and the carbon gets whatever is. C3H8g 5O2g 3CO2g 4H2Og.
Before we balance the incomplete combustion of pentane lets remember first that the balanced equation for the complete combustion for pentane will be written as follows. Incomplete combustion. Good signs that youre dealing with a combustion reaction include.
I assume you are referring to the complete combustion of a hydrocarbon which produces carbon dioxide and water vapor. Combustion or burning is a high-temperature exothermic redox chemical reaction between a fuel the reductant and an oxidant usually atmospheric oxygen that produces oxidized often gaseous products in a mixture termed as smokeCombustion does not always result in fire because a flame is only visible when substances undergoing combustion vapourise but when it does a flame is a. Click to see full answer.