Awesome Incomplete Combustion Of Butane

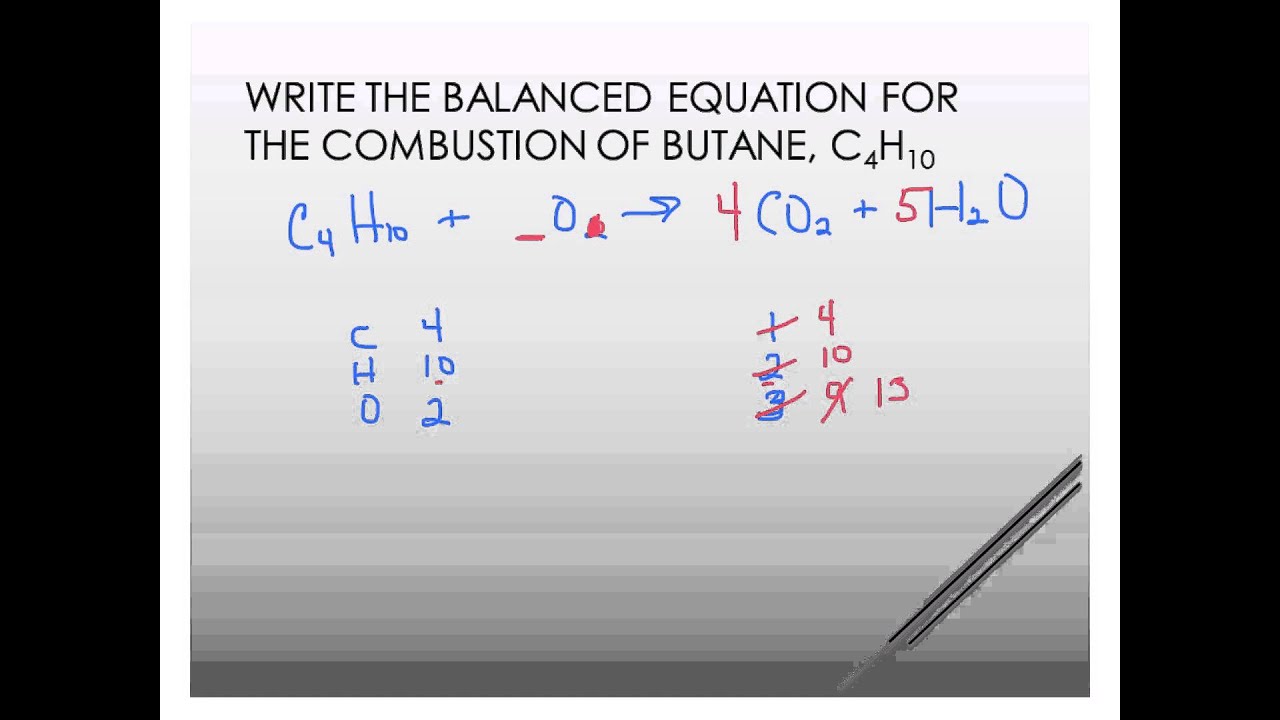
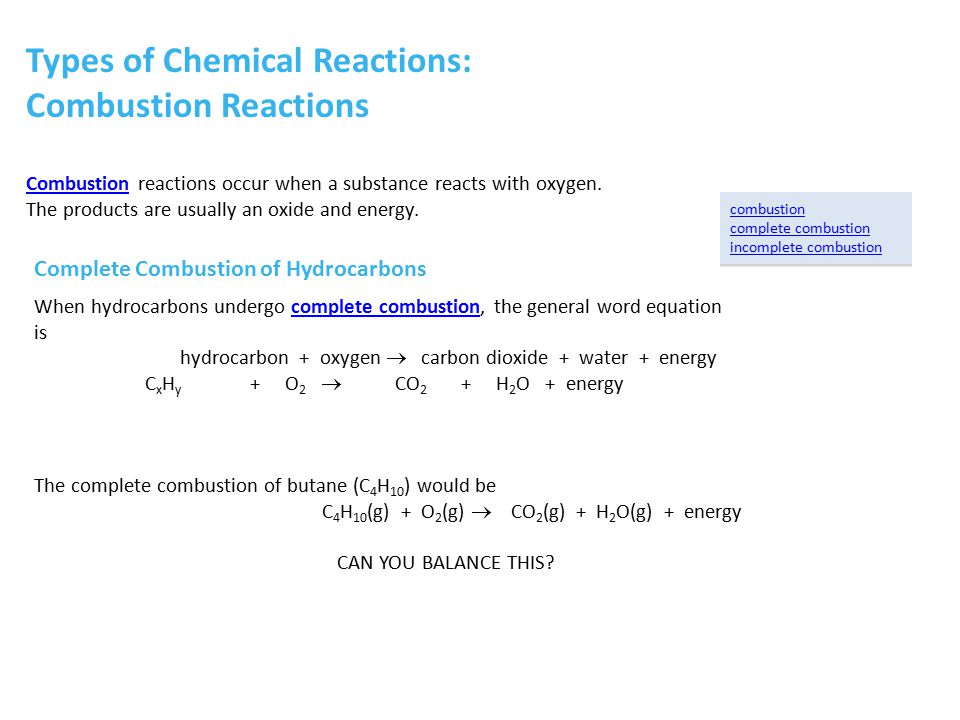
Butane C4H10 reacts with oxygen O2 to make carbon dioxide CO2 and water H2O.
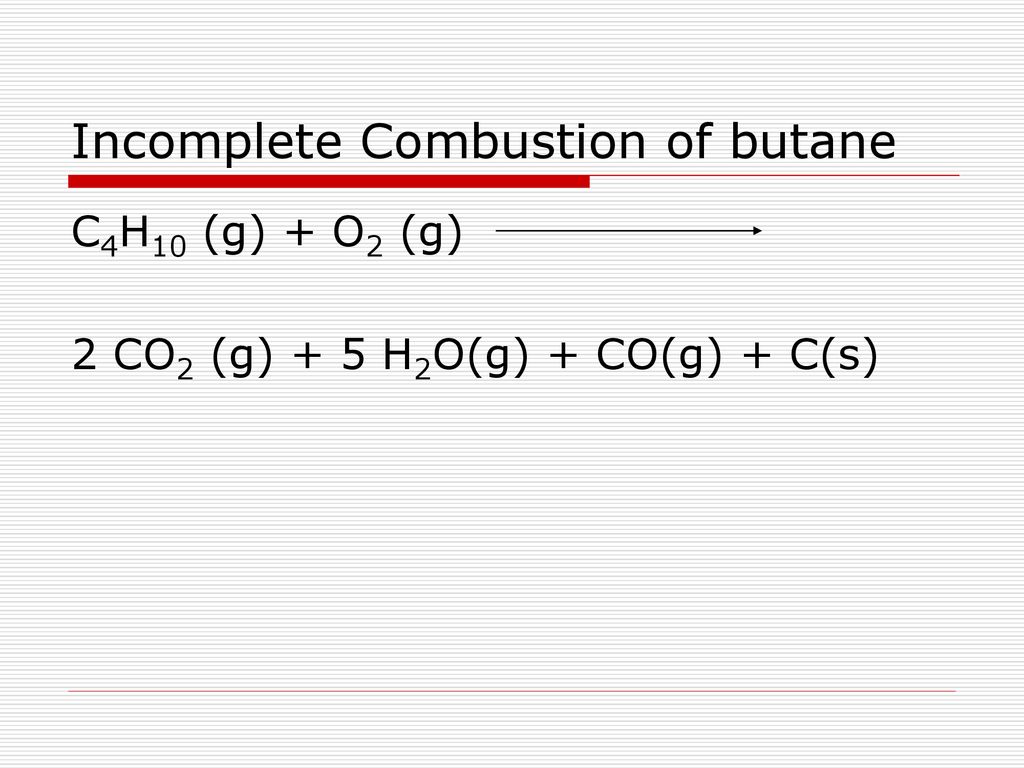
Incomplete combustion of butane. Butane dioxygen carbon dioxide water. C4H10 O2 CO2 H2OFind a balanced chemical equation for this reaction. For the question above use the following instructions - iForm the system of linear equations.
What are the differences. Find an answer to your question An equation for the incomplete combustion of butane in oxygen is Amena2000 Amena2000 03252017 Chemistry High School answered expert verified. Chemistry tutor 1947 Views See similar Chemistry GCSE tutors.
2 C3H8 9 O2 4 CO2 2 CO 8 H2O Heat. A complete combustion of butane b complete combustion of ethanol c incomplete combustion of cyclopentane Cracking Write a balanced molecular equation showing the formation of ethene from the cracking of hexane. Complete combustion of butane will produce carbon dioxide and water but incomplete combustion not enough oxygen will produce carbon monoxide.
In general for incomplete. Water is still produced but carbon monoxide and carbon are produced instead of carbon dioxide. Complete combustion does NOT give carbon monoxide or sootCheck me out.
If there is not enough oxygen incomplete combustion will occur so CO carbon monoxide will be formed carbon will form soot and unburnt hydrocarbons will be releasedThis is dangerous as CO is a toxic gasAlso soot causes global dimming. There tends to be more incomplete combustion with long chain hydrocarbons than short chain ones. General equation for incomplete combustion reaction follows.
If not enough oxygen is present for complete combustion incomplete combustion occurs. The hydrogen atoms react with oxygen to form water. If I do exactly as problem says I guess it.