Awesome Incomplete Combustion Reaction Equation
This is mostly thermal energy but.
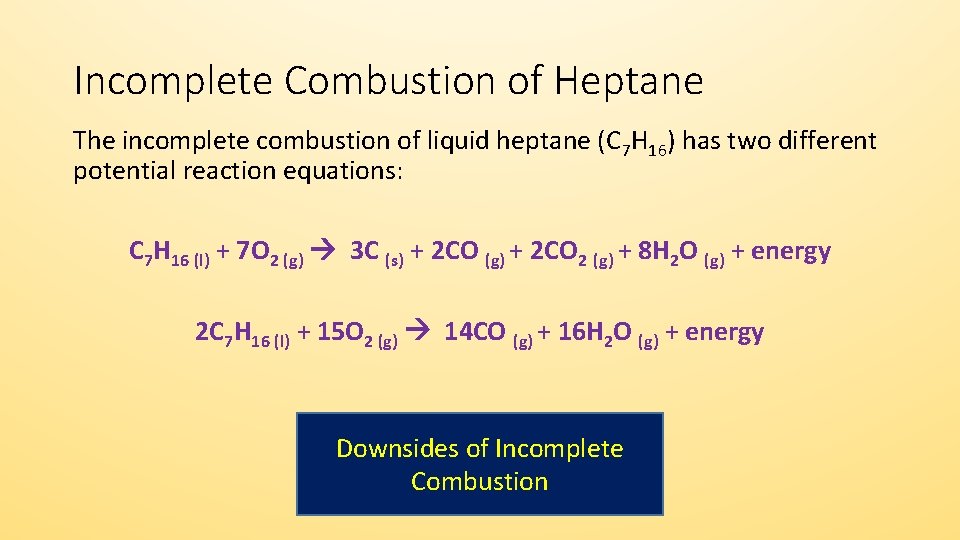
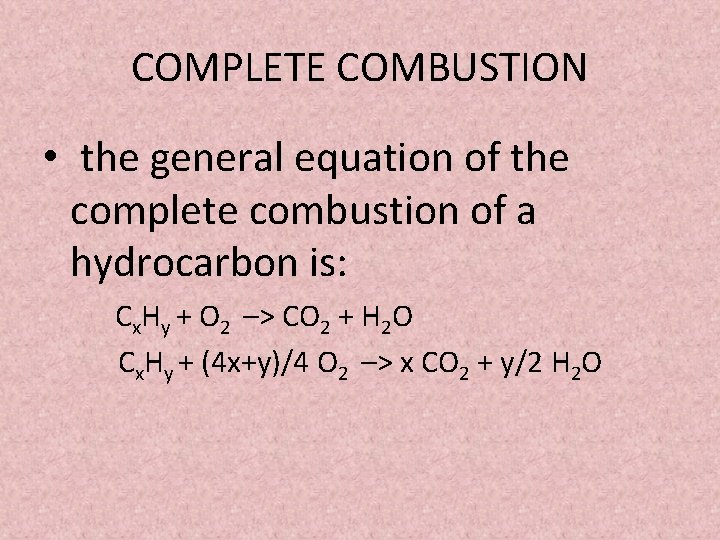
Incomplete combustion reaction equation. Combustion may be complete or incomplete. For example CH4 C2H4 H2S are all gases which can burn but will have different equations for complete combustion. 2 C3H8 9 O2 4 CO2 2 CO 8 H2O Heat.
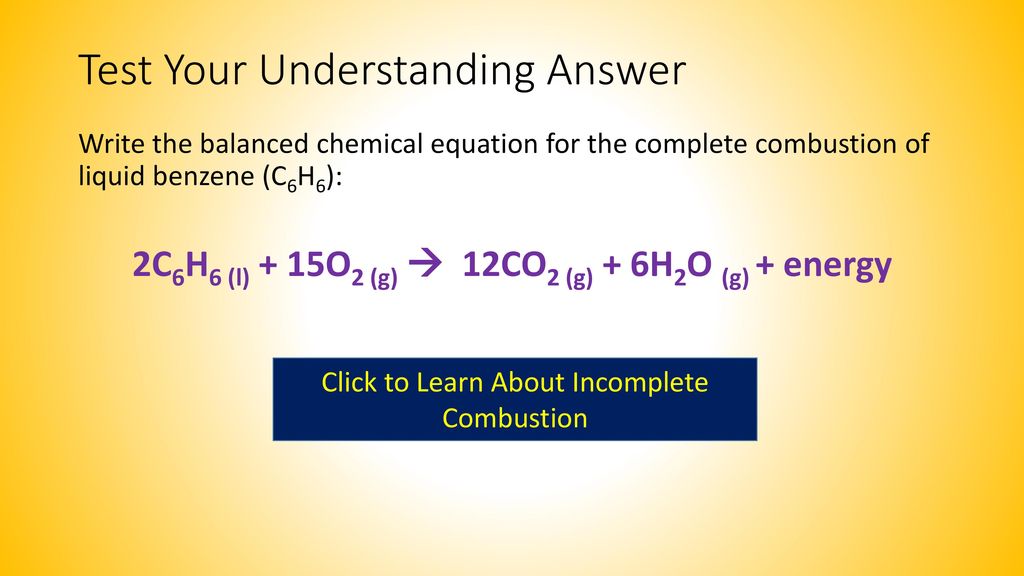
It is quite important that you can write properly balanced equations for these reactions because they often come up as a part of thermochemistry calculations. 2 C6H6 l 15 O2 g -- 12 CO2 g 6 H2O g Calculate the percent yield of the reaction if the combustion of 136 g C6H6 MW 7812 gmol with excess O2 MW 3200 gmol produced 401 g CO2 MW 4401 gmol 5 years ago. The equation for incomplete combustion of propane is.
It is possible to give an equation for complete combustion but the equation depends on what is being combusted. The word equation for combustion is. Hydrocarbons readily burn or undergo combustion reactions.
Fuel O2 CO2 H2O. Recognizing the characteristics and balancing incomplete combustion reactions. Gasification is an incomplete combustion of the fuel.
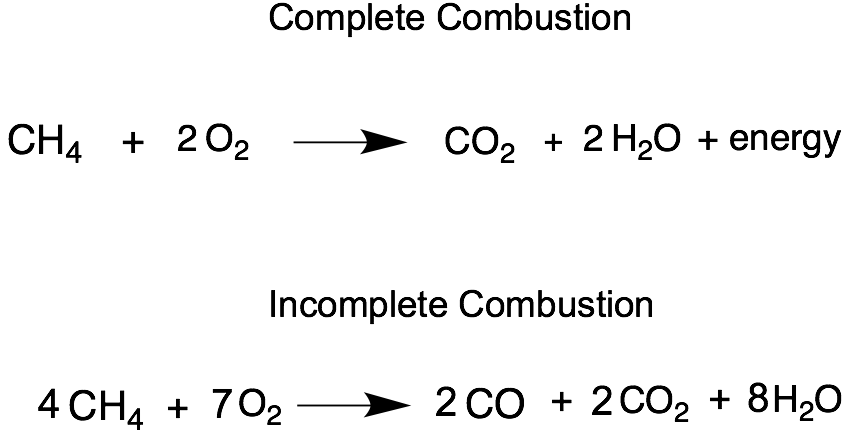
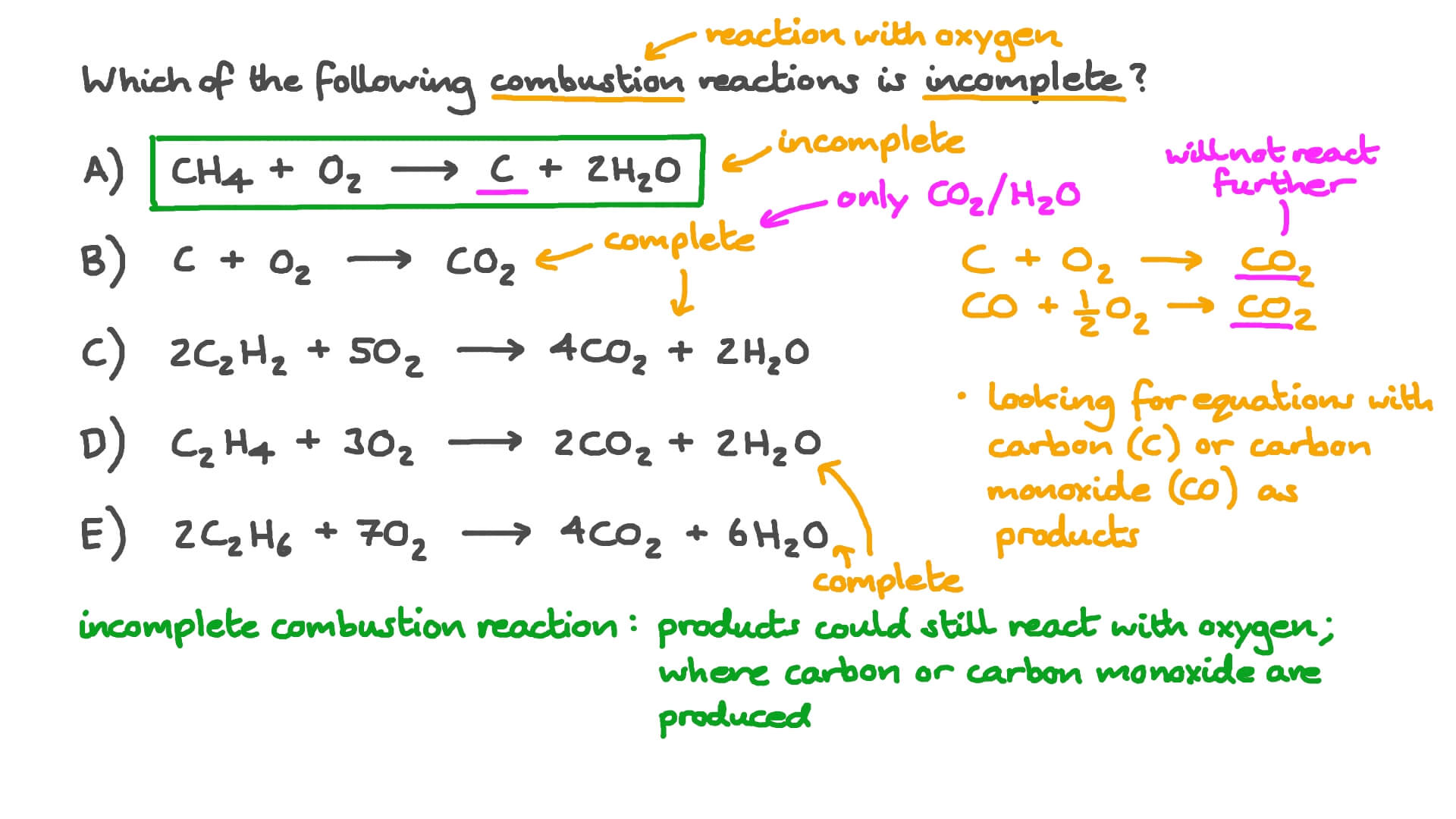
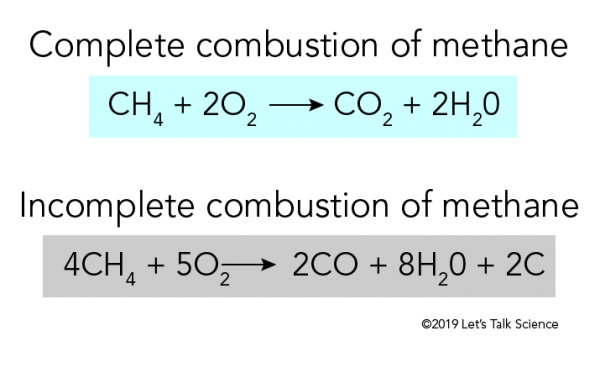
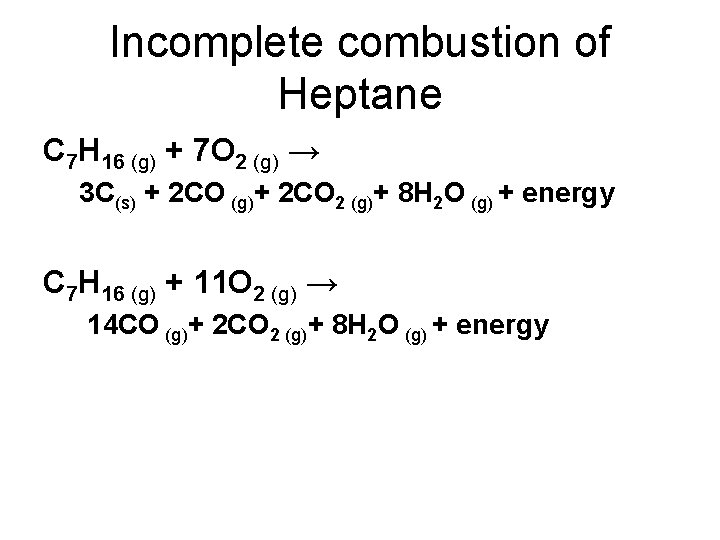
Complete combustion of fossil fuels results the production carbon dioxide and water. Incomplete Combustion of Methane. A Incomplete Combustion Equation - Multiple carbon products Hydrocarbon Oxygen Carbon Carbon monoxide water When writing equations with incomplete combustion it is advisable to include only one carbon product otherwise there will be multiple solutions to the equation.
Incomplete combustion is also a reaction between oxygen and fuel but the products are carbon monoxide water and carbon. Click to see full answer. 2C3H 8 g 7O2 g 2C s 2CO g 2CO2 g 8H2O g 8.