Beautiful Iron Corrosion Formula

Hydrated ironThe primary corrosion product of iron is FeOH2 or more likely FeOnH2O but the action of oxygen and water can yield other products having different colors.
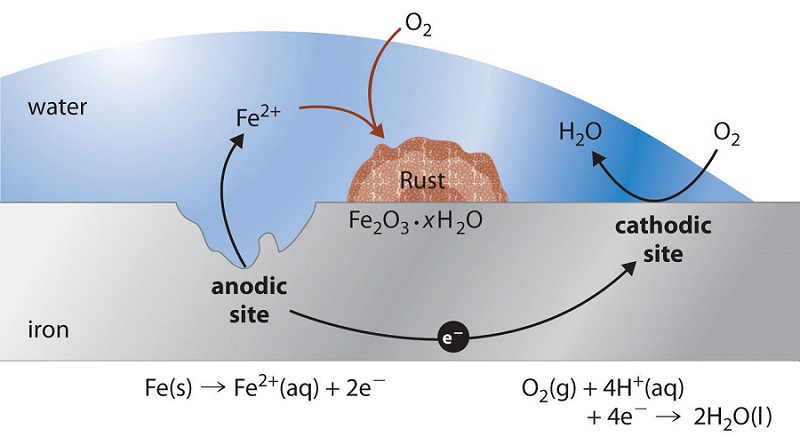
Iron corrosion formula. The rusting of iron. Corrosionpedia explains Iron Corrosion. The chemical reaction in which iron Fe combines with oxygen O2 to form rust or iron oxide Fe2O3.
Rust is the common name for iron oxideThe most familiar form of rust is the reddish coating that forms flakes on iron and steel Fe 2 O 3 but rust also comes in other colors including yellow brown orange and even greenThe different colors reflect various chemical compositions of rust. The Rusting of Iron. To calculate the corrosion rate from metal loss use.
Rust is apparently a hydrated form of iron IIIoxide. Fe2O3H2O hydrous ferrous oxide sometimes written as FeOH3 is the principal. Corrosion of Iron Demonstration and Inquiry Introduction Rust is expensive.
Rusting is the corrosion of iron and readily occurs in the alloy steel. Corrosion is a galvanic process by which metals deteriorate through oxidationusually but not always to their oxides. What kinds of chemical treatments surface coatings or combinations of metals will.
The equation for this reaction is. Points of stress acts in the nail allow the iron to be easily oxidised. Corrosion behaviour of iron and iron-manganese sintered structures in a medium simulating a human body fluid environment.
Iron is mentioned numerous times in the Old Testament of the Bible. Iron III oxide or ferric oxide where the iron atom exhibits an oxidation state of 3. The formula is approximately Fe 2 O 3 3 2 H 2 O although the exact amount of water is variable.