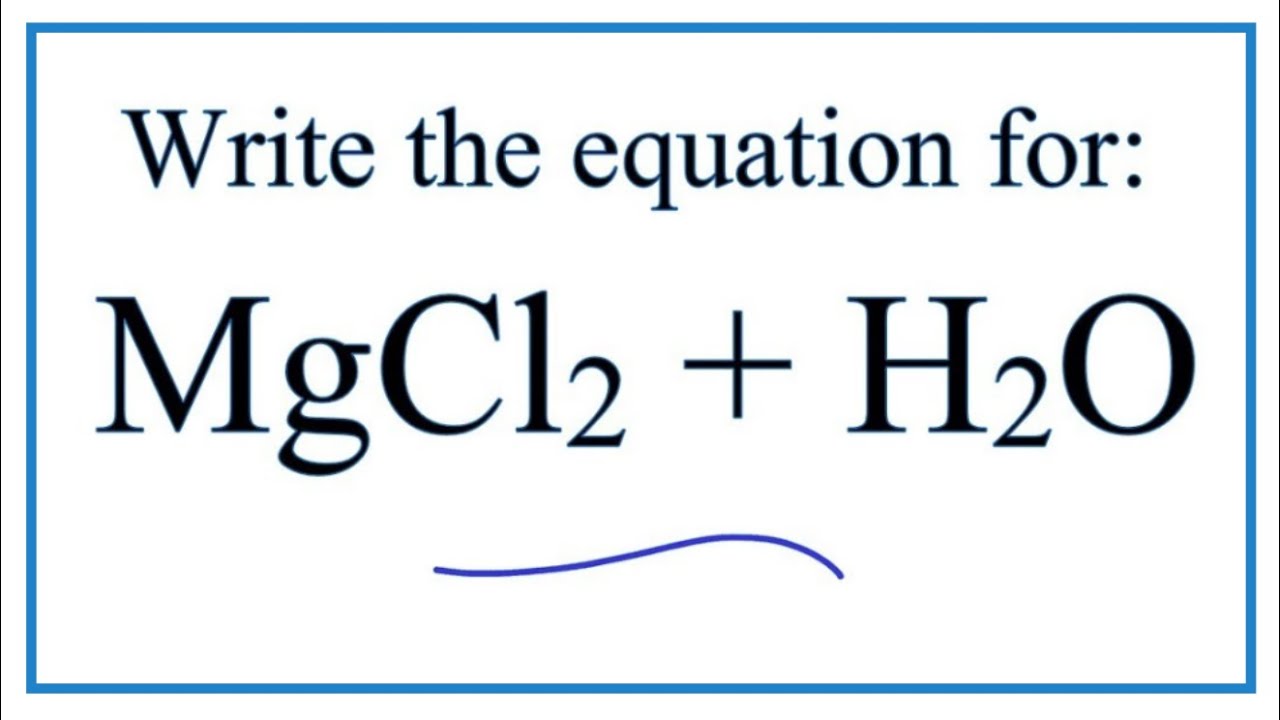Top Notch Magnesium Chlorine Reaction

It has also been used as a cathartic and in alloys.
Magnesium chlorine reaction. About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy Safety How YouTube works Test new features Press Copyright Contact us Creators. An inorganic compound consisting of one magnesium and two chloride ions. Magnesium Chloride Reactions In the Dow process magnesium hydroxide reacts with hydrochloric acid to regenerate magnesium chloride.
In this paper is reported a novel method to synthesize nesquehonite MgCO3 x 3H2O via reaction of a flux of CO2 with Mg chloride solution at 20-2 degrees C. Data sources include IBM Watson Micromedex updated 2 Aug 2021 Cerner Multum updated 3 Aug 2021. The compound is used in medicine as a source of magnesium ions which are essential for many cellular activities.
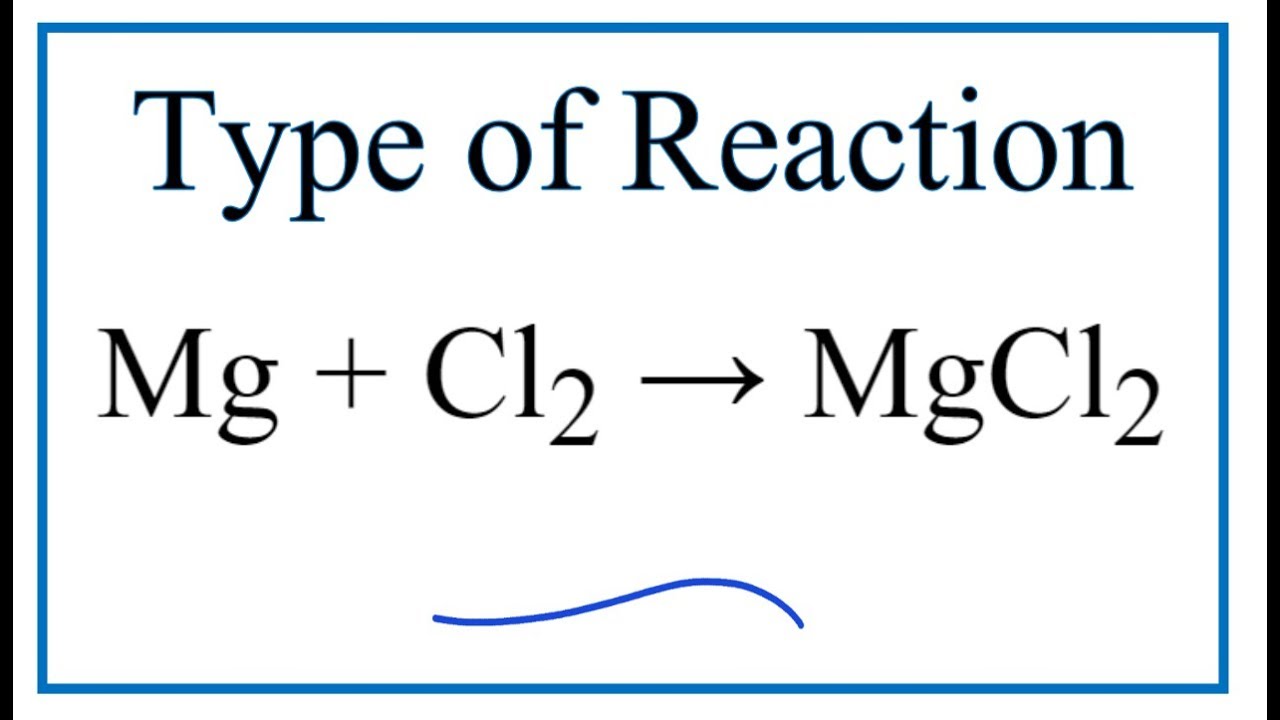
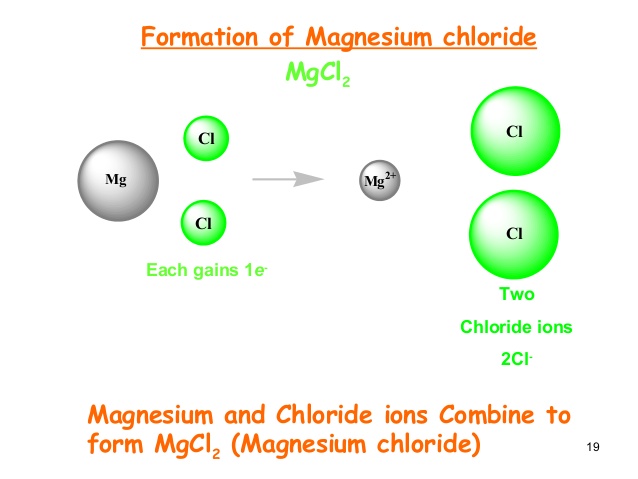
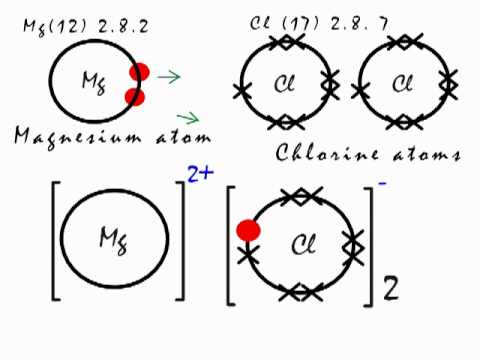
Magnesium Mg reacts with chlorine gas Cl2 to produce magnesium chloride Mg2Cl2. Mg OH2 s. To obtain full outer shells magnesium must lose two electrons and chlorine.
MgBr₂ Cl₂ ----- MgCl₂ Br₂ As you can see products are Magnesium chloride and Bromine. The full characterization of the product of syn. The reaction rate is rapid with carbonate deposition almost complete in about 10 min.
The water is evaporated leaving solid magnesium chloride. Reaction between acid and base is neutralisation as it forms the products - salt and water and the salt is neutral Neither acidic nor alkali but at pH 7 equal concentration of hydrogen and hydroxide ions. C l X 1 2 I X 2 1 2 C l X 2 I X.
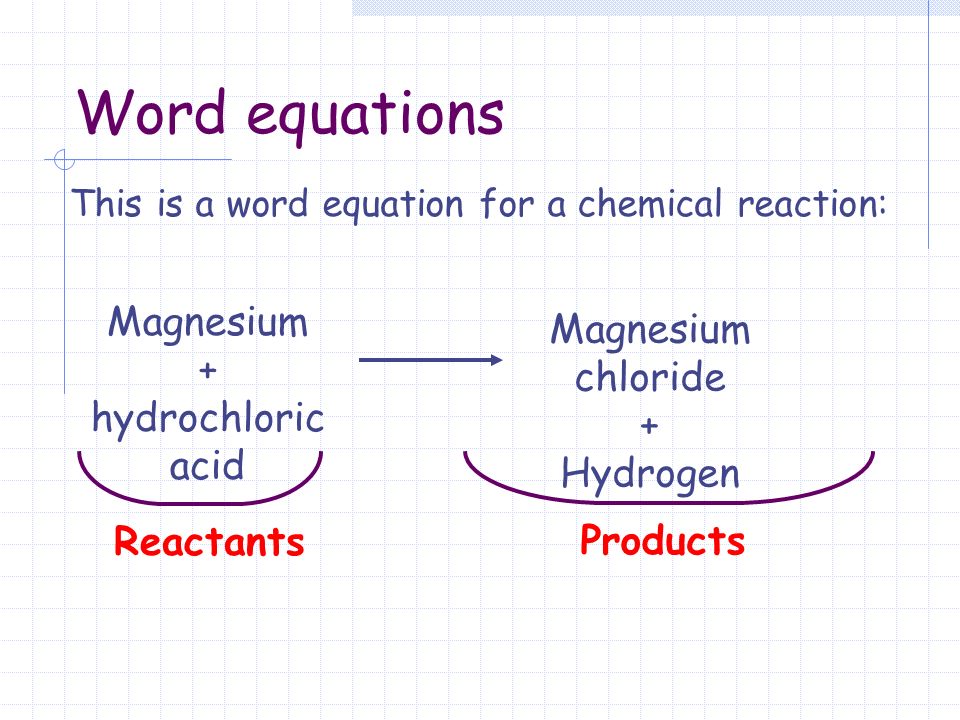
One may also ask how does magnesium and chlorine combine to form magnesium chloride. The reaction is as follows. In the Dow process magnesium chloride is regenerated from magnesium hydroxide using hydrochloric acid.