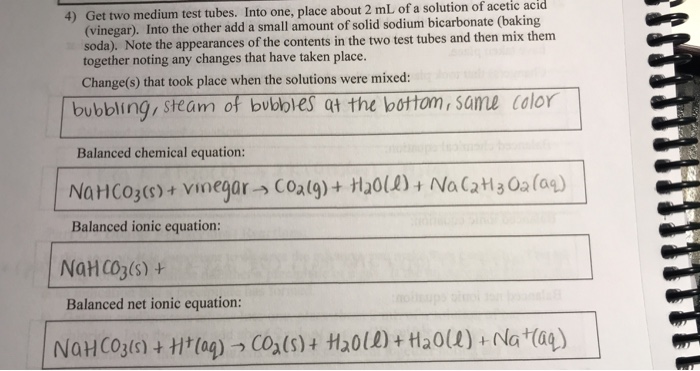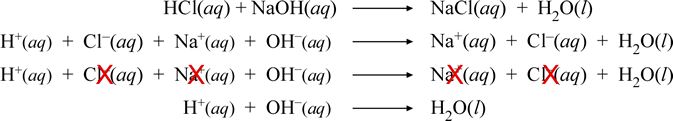Ace Net Ionic Equation For Baking Soda And Vinegar
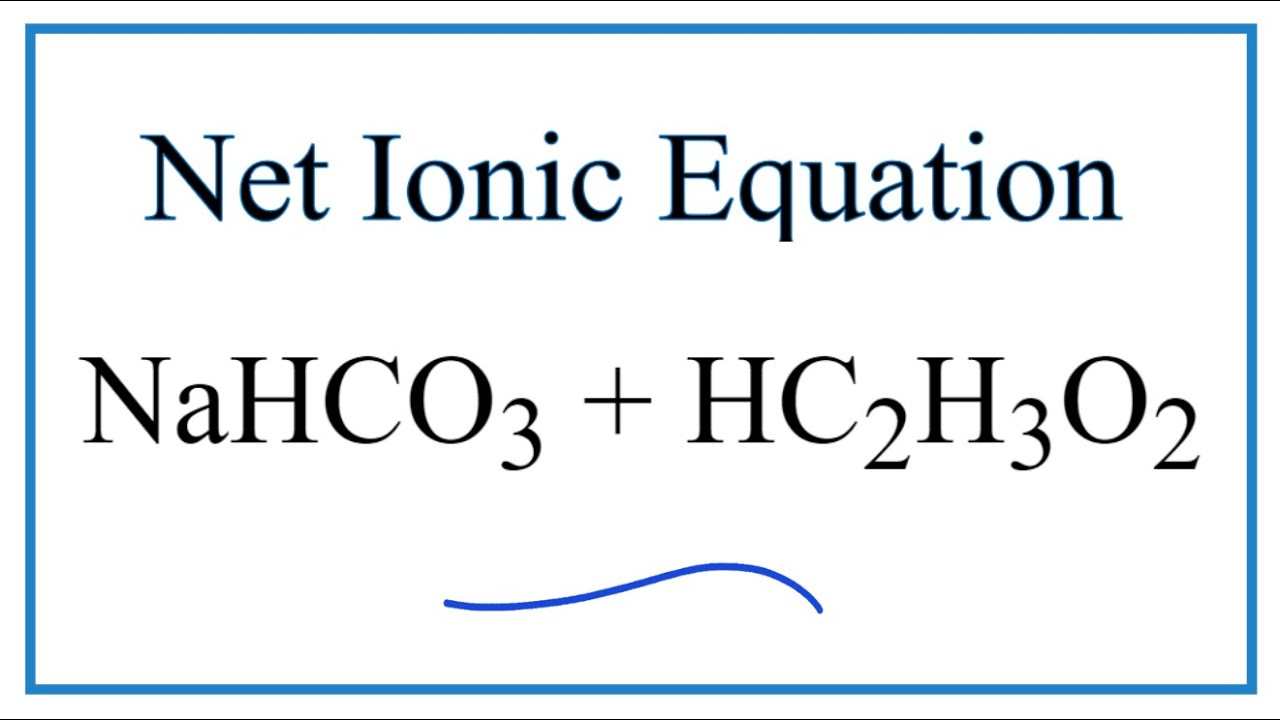
The major component in baking soda is NaHCO3sodium bicarbonate f Write the overall equation and the net ionic equation for the process.
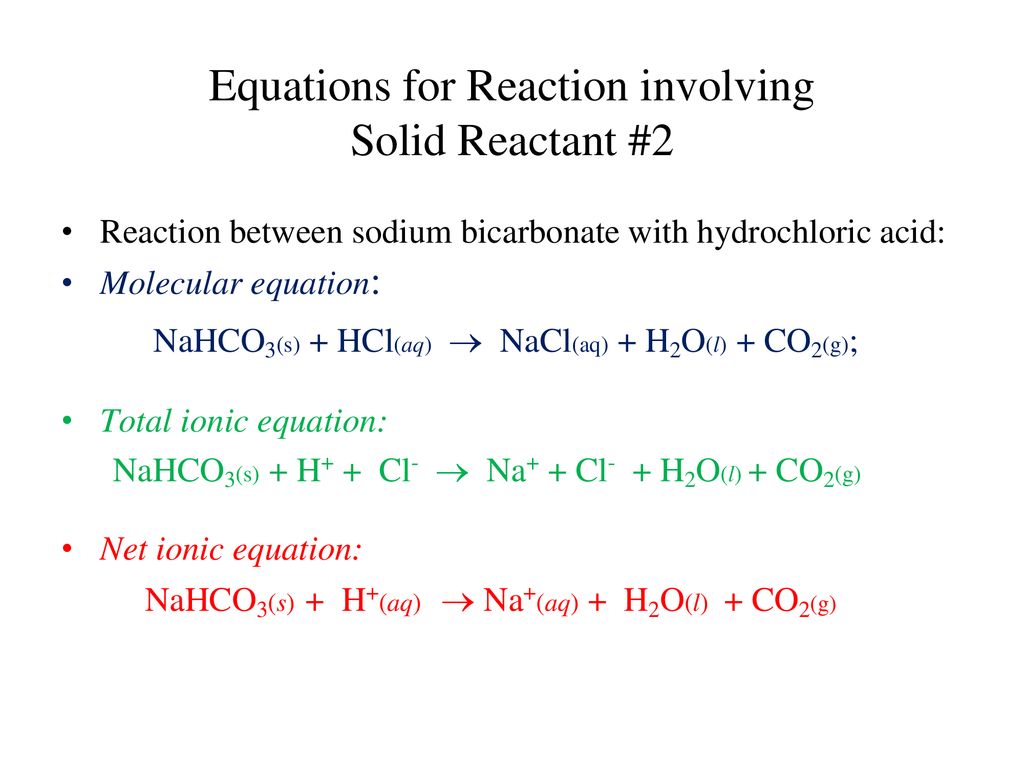
Net ionic equation for baking soda and vinegar. The answer I got was H aq HCO3- aq H2O l CO2 g but apparently. The reaction between baking soda and vinegar actually occurs in two steps but the overall process can be summarized by the following word equation. For each antacid write a balanced molecular equation and net ionic equation that shows how each antacid works.
The chemical equation for the overall reaction is. This reaction is often used in volcano demonstrations. NaHCO3aq CH3COOHaq CO2g H2Ol CH3COONaaq In the first 2 flasks the limiting reagent is the baking soda.
Which antacid will make you burp. Start date Oct 29 2006. See Practice Problems 4 and 5.
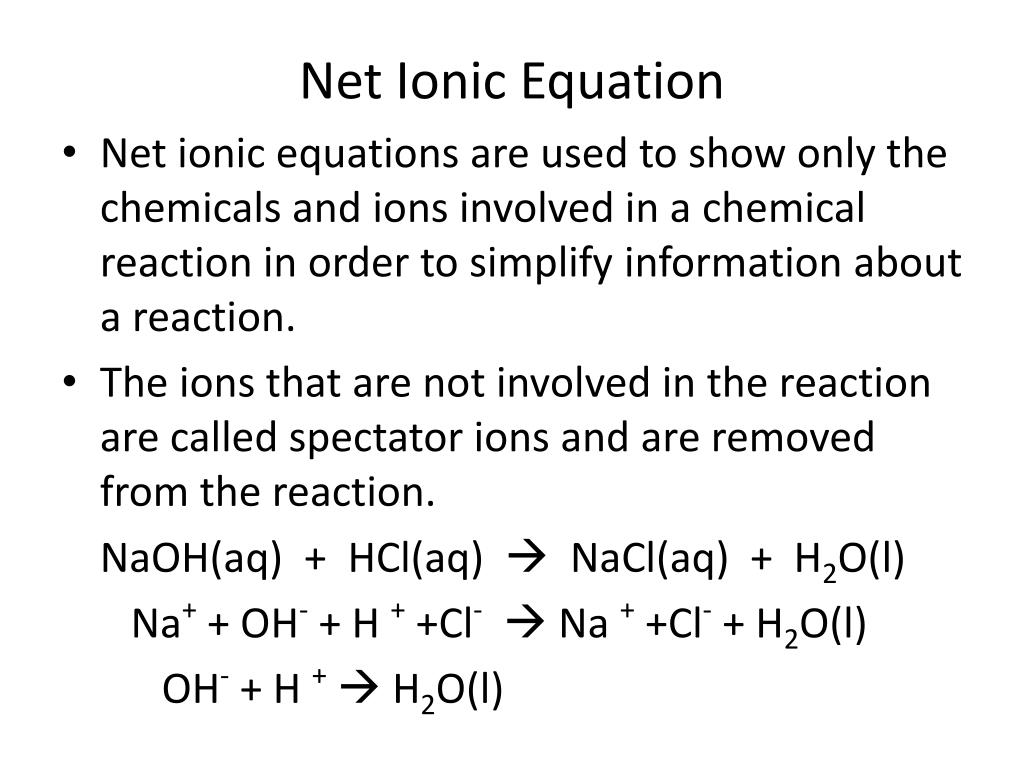
When vinegar CH3COOH and baking soda NaHCO3 react the following reaction occurs. Write a balanced net ionic equation for the reaction of aqueous solutions of baking soda NaHCO3 and acetic acid. The equilibrium shifts tow.
Therefore as you add more baking soda the balloon gets bigger. This chemistry video tutorial discusses the reaction between baking soda and vinegar. Asked Jun 29.
For fresh cabbage juice 4-5 leaves of fresh red cabbage water 500 mL denatured alcohol blender funnel cheesecloth or paper towel 600 mL beaker. One example is the reaction between vinegar and baking soda. Any of the following.