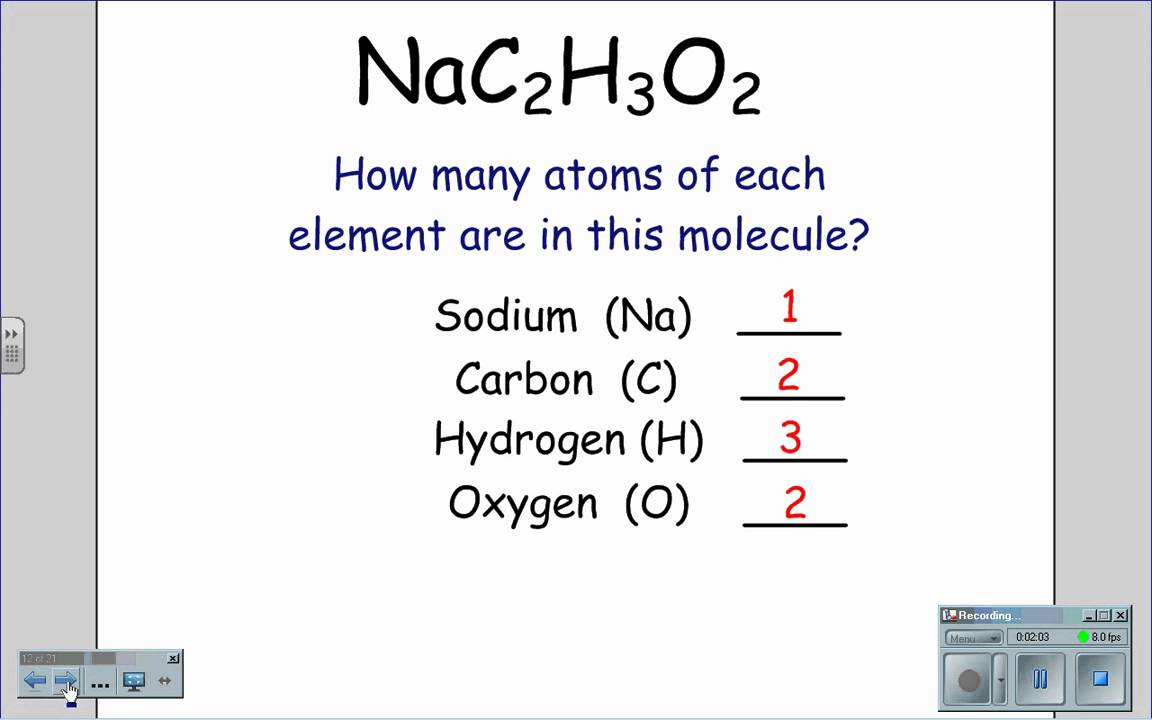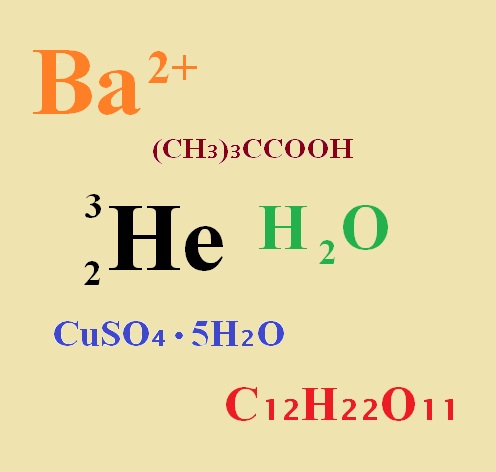Divine What Do The Subscripts Mean In A Chemical Formula

ALE Page 1 of 6 Formulas of Ionic and Molecular Compounds Dr.
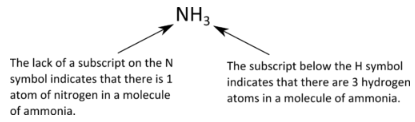
What do the subscripts mean in a chemical formula. What does subscript s mean in chemistry. Formulas of Binary Ionic Compounds Look at the Periodic Table in your textbook that shows the elements as metals nonmetals and metalloids. The numbers appearing as subscripts in the chemical formula indicate the number of atoms of the element immediately before the subscript.
If no subscript appears one atom of that element is present. The subscript lower represents the number of each atom while the superscript higher represents the charge on a given atom. If no subscript appears one atom of that element is present.
What do subscripts mean. Subscripts are commonly used in chemical formulas. Subscripts in Chemical Formulas Subscripts are important numbers in formulas especially when youre trying to understand how many atoms of an element are present.
If for example they weighed out 1 g of material and performed elemental analysis for each element then the subscripts you see would be the ratio of that element. These subscripts indicate the number of atoms of each given element that are contained in the. A coefficient before a chemical formula represents that many units of the molecule.
Atoms of that element. A scientist would write the formula for water H2O so that the 2 appears lower and smaller than the letters on either side of it. The numbers appearing as subscripts in the chemical formula indicate the number of atoms of the element immediately before the subscript.
Chemical formulas use letters and numbers to represent chemical species ie compounds ions. A subscript is a number to the right and below the abbreviation of an element that indicates the number of elements that are present. What do subscripts mean.