Beautiful Work What Is The Balanced Equation For Rust
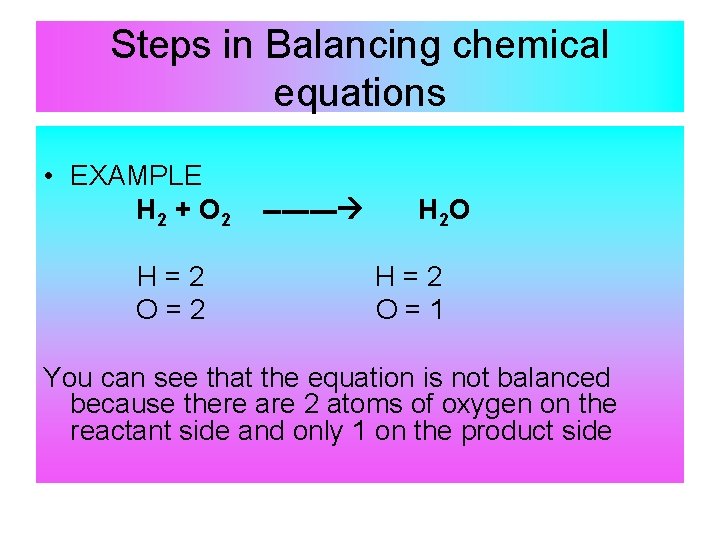
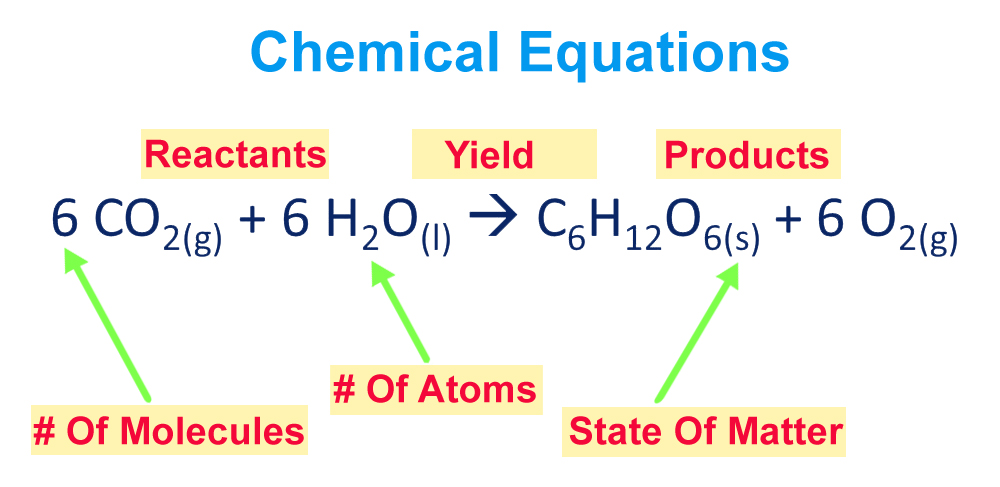
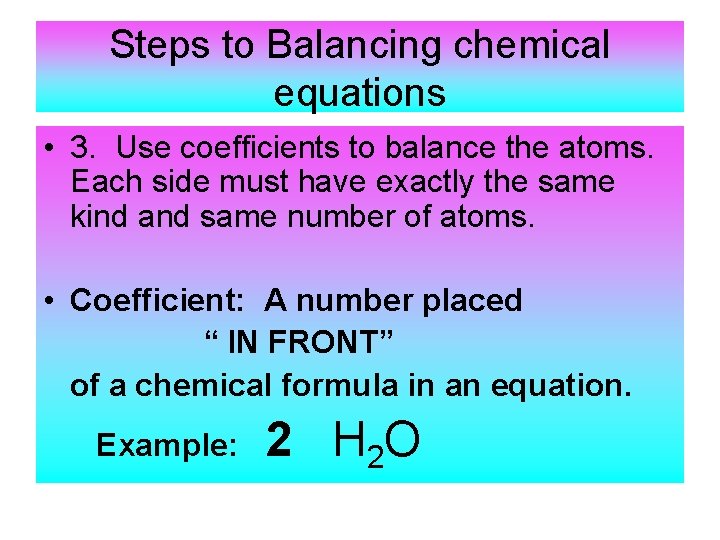
The equation in which the number of atoms of all the molecules is equal on both sides of the equation is known as a balanced chemical equation.
What is the balanced equation for rust. Fe O2 Fe2O3 is the unbalanced form of chemical reaction. Four moles of iron metal react with three moles of oxygen gas to produce two moles of iron III oxide. Water is also required for this reaction to occur but because the total amount of water does not change it is not included in the equation.
Oxidation of Solid Iron Its common knowledge that rust occurs when you leave water on a. The formation of rust requires iron water and oxygen. How do you make Fe2O3.
Fe OH3 dehydrates to produce Fe2O3nH2O s or rust. Rust iron III oxide forms when iron and oxygen react. Click to see full answer People also ask what is the balanced equation for Iron III.
Although its a complex process the chemical equation is simply 4Fe 3O2 6H2O 4Fe OH3. Rust is the common name for iron oxideThe most familiar form of rust is the reddish coating that forms flakes on iron and steel Fe 2 O 3 but rust also comes in other colors including yellow brown orange and even greenThe different colors reflect various chemical compositions of rust. The representation of a chemical reaction in the form of substances is known as a chemical equation.
Fe2O3 CO Fe CO. Which is the correctly balanced equation for the reaction of rust Fe2O3 and hydrochloric acid HCl. Which is the correctly balanced equation for the reaction of rust fe2o3 and hydrochloric acid hcl.
Is Fe2O3 3co 2fe 3co2 a balanced equation. Fe2o3 hcl fecl3 h2ofe2o3 3 hcl 2 fecl3 h2ofe2o3 6 hcl 2 fecl3 3 h2ofe2o3 3 hcl 2 fecl3 3 h2o Answers. How long does it take for iron to rust.