Glory Zinc Reacts With Sulphuric Acid

The reaction is described by the following equation.
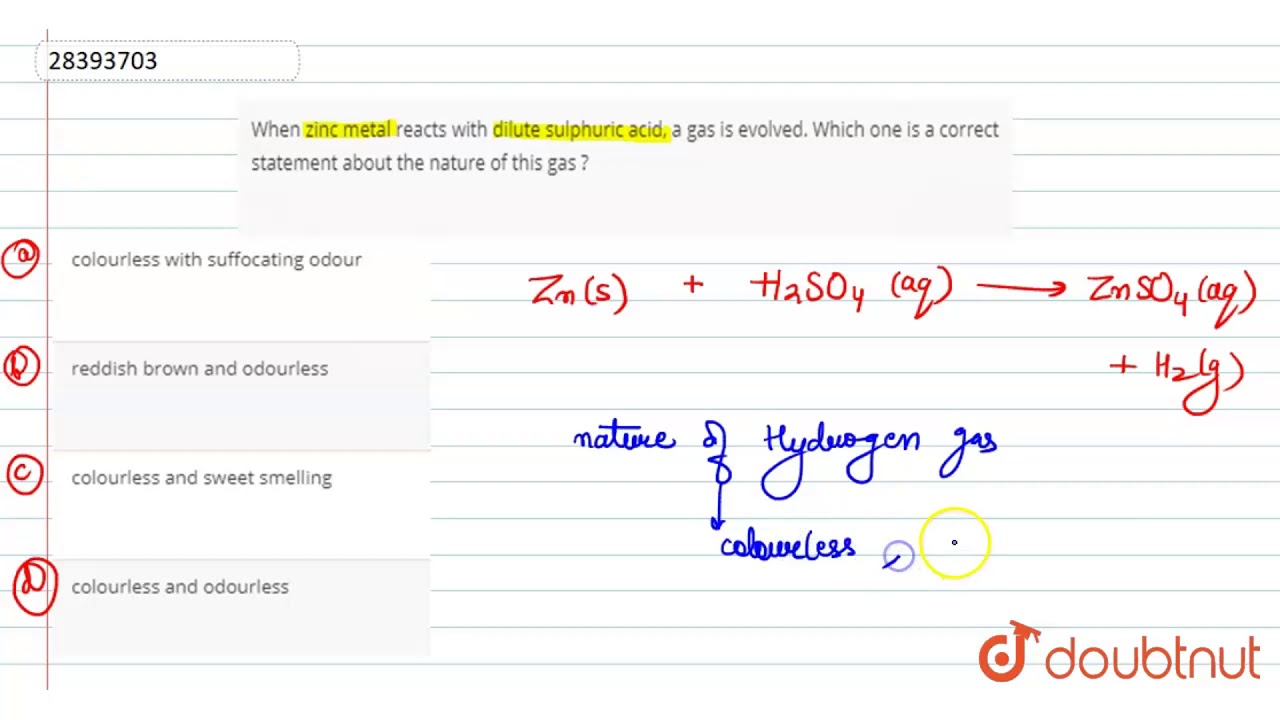
Zinc reacts with sulphuric acid. Zinc reacts with H2SO4 to form Zincsulphate and hydrogen gas is also liberated. Zinc reacts with dilute sulphuric acid to form zinc sulphate and hydrogen gas is evolved. This is a single displacement reaction of a non-metal by a metal.
A What volume of hydrogen gas is produced at STP when 065g of zinc reacts completely with excess hydrochloric acid. What is the type of salt created by Sulphuric acid. Zn H2SO4 ZnSO4 H2.
Zn s H 2 SO 4 g ZnSO 4 s H 2 Zinc dil. The balanced equation for this reaction is. What happens when potassium reacts with acid.
How do you make coffee less acidic. Zinc dissolution in sulphuric acid has all the characteristics of acorrosion process whose speed is controlled by cathodic reaction that isreduction of Hion and can be accelerated if. The reaction would be MUCH more rapid with hydrochloric acid which follows the same stoichiometry What happens when zinc metal was mixed with hydrochloric acid.
This is a single displacement reaction of a non-metal by a metal. A Dilute sulphuric acid reacts with zinc granules. The reaction produces moles of zinc sulfate and moles of water.
Zinc reacts with dilute sulphuric acid to produce hydrogen gas H 2 and zinc sulphate. Zinc reacts with dilute sulphuric acid to form zinc sulphate and hydrogen gas is evolved. 1 mole of metal reacts with 1 mole of acid.