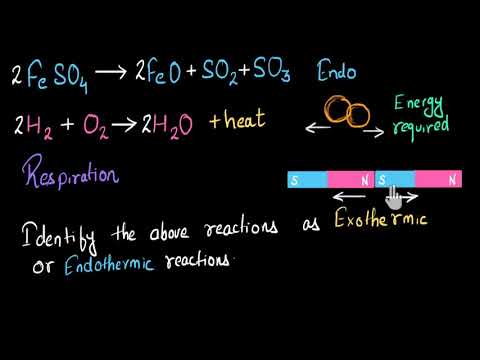Supreme Example Of Exothermic Reaction Equation

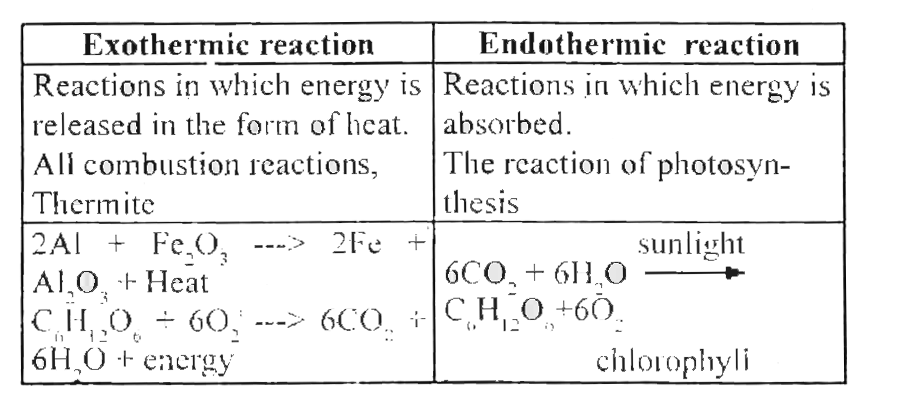
So an exothermic reaction results in the chemical product and a release of energy.
Example of exothermic reaction equation. The following equations describe the combustion of a hydrocarbon such as petrol C8H18 C 8 H 18. In an exothermic reaction change in enthalpy ΔH will be negative. The burning of hydrogen in hydrogen-oxygen fuel cells is a type of exothermic reaction as it undergoes combustion and produces an electric charge The burning of natural gas methane is also an example of an exothermic reaction.
When the temperature reaches below 0 ⁰C or 32 ⁰F the water changes from liquid to solid freezing. For example the combustion reaction of butane the gas we burn in the kitchen has an enthalpy variation of -11823 kcal mol. The chemical equation can be.
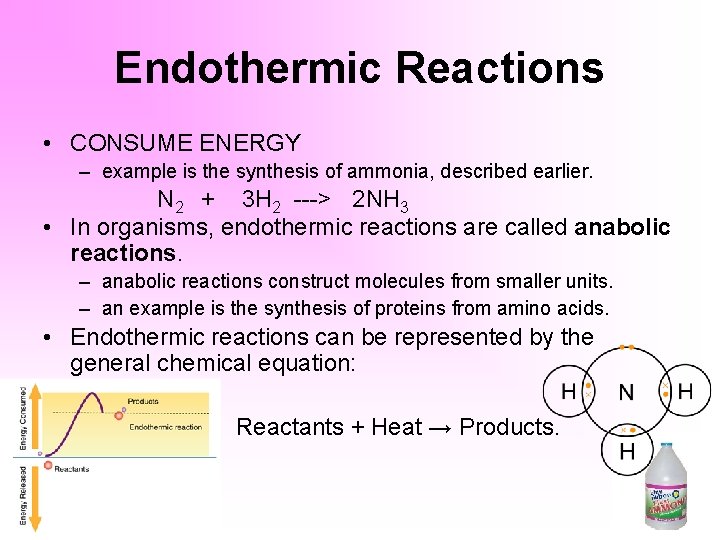
Examples Of Exothermic Reaction Lab Report. Another way to think of exothermic versus endothermic reaction is by chemical bonds. Reactants products energy.
These are reactions that transfer energy to the surroundings ie the energy exits from the reaction hence the name exothermic. Any combustion reaction is always involved with molecular oxygen O2. Remember that chemical reactions involve a reorganization of atoms between substances with breakage or formation of.
Any reaction that has a negative AH read delta H will be exothermic. An endothermic reaction requires energy while an exothermic reaction releases energy. An exothermic reaction is defined as a reaction that releases heat and has a net negative standard enthalpy change.
Given below is the reaction in the form of an equation CH₄2O₂CO₂2H₂O. There are many useful examples of exothermic reactions and processes in everyday life. What are 3 exothermic reactions.