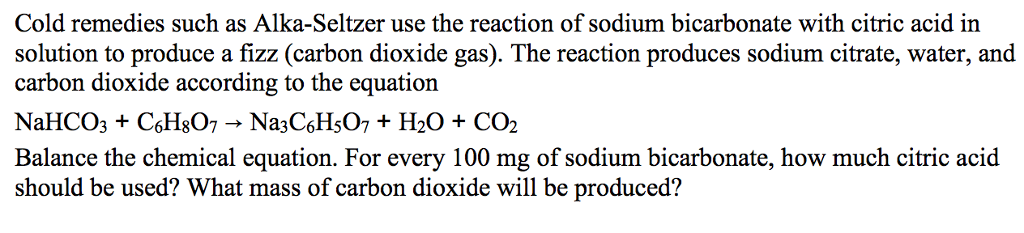Looking Good Alka Seltzer Water Reaction Equation

Alka seltzer equation jennarocca and water chemical for balanced equations you decide which substance limits hot cold exploring the gas laws rt table 10 1 chemistry of chem13 reaction rate experiment with.
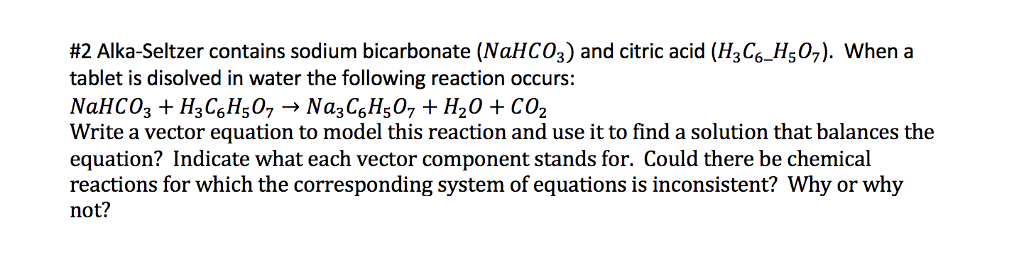
Alka seltzer water reaction equation. When you drop the tablet in water the acid and the baking soda react this produces the fizz. Reaction 42 I New bubbies appeared The Alka seizer dissolved in water - the test tube got cold 3. C6H8O7 3NaHCO3 - Na3C6H5O7 3CO2 3H2O.
Alka-Seltzer tablets contain citric acid and sodium bicarbonate. Reaction 1 pheat was released as I was mixing it up the water star - The liquid went from getting warmly white test tube tuis warm 2. Water dissolves these two substances and allows them to react and form carbon dioxide gas as shown below.
Alka seltzter and water reaction can you guess which jar contains the warmest water. Citric acid aq Sodium bicarbonate aq - Carbon dioxide water Sodium citrate H 3C6H 5O7aq 3N aH CO3aq 3CO2g 3H 2Ol N a3C6H 5O7aq. They could have a mixture of sodium bicarbonate separated by some type of dry acid on the other side.
In the presence of water citric acidC6H8O7 and sodium bicarbonate NaHCO3 aka baking soda react to form sodium citrate Na3C6H5O7 water and carbon dioxide CO2 NaHCO3C6H8O7 H2ONa3C6H5O7CO2 C6H8O7 3NaHCO3 Na3C6H5O7 3CO23H2O 286K views. HC 2 H 3 O 2 NaHCO 3 NaC 2 H 3 O 2 H 2 O CO 2 An Alka-Seltzer tablet contains aspirin citric acid and sodium bicarbonate. In fact the molecule holding the key to the relief of an Alka-Seltzer tablet is the bicarbonate ion.
The reaction is. Thus our frightening equation above can be dramatically simplified to. They produce gas fizzing when dissolved in water.
To take the tablets theyre fully dissolved in water where they famously undergo a chemical reaction that. NaCO3 HCl H2O - CO2 gas NaCl table salt H2O did not bother to balance the equation. Sodium acetylsalicylate sodium bicarbonate sodium citrate drug combination.