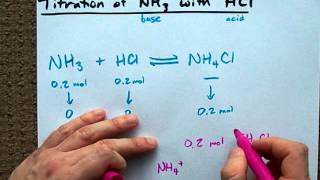Top Notch Ammonia React With Hcl

Hydrochloric acid reacts with ammonia to form ammonium chloride a salt.
Ammonia react with hcl. Ammonia solution reacts with hydrochloric acid a solution of hydrogen chloride in water in a similar way. If you mix together a solution of ethanoic acid and a solution of ammonia you will get a colorless solution of ammonium ethanoate. I mean really what.
One may also ask what is the chemical reaction for ammonia. HCl NH3 NH4Cl A student is titrating 50 mL of 032 M NH3 with 05 M HCl. What happens when ammonia reacts with hydrochloric acid.
Part of NCSSM CORE collection. Ammonium chloride appears to sublime upon heating but actually decomposes into ammonia and hydrogen chloride gas. Ammonia is a weak base that reacts with hydrochloric acid forming a compound called ammonium chlorideDuring this chemical reaction hydrochloric acid donates a proton to ammonia meaning the former compound acts as a Bronsted-Lowry acid while the latter is a Bronsted-Lowry base.
Ammonium chloride pyrolyses and reforms into ammonium chloride smoke after cooling. NH3g HCl g NH4Cls. The reaction equation between ammonia NH3 and hydrochloric acid HCl is written as follows.
MathNH_3 HCl H_2Omath how. Ammonia is a weak base that reacts with hydrochloric acid forming a compound called ammonium chloride. The reaction equation between ammonia NH3 and hydrochloric acid HCl is written as follows.
The theft leaves chloride alone and negative. This video shows the gas phase acid base reaction of Ammonia and Hydrochloric Acid. Why doesnt the reaction of aqueous ammonia and HCl solutions produce water.