Fantastic Sodium With Cold Water Equation
Students investigate this endothermic reaction.
Sodium with cold water equation. In the presence of water citric acid and sodium bicarbonate aka baking soda react to form sodium citrate water and carbon dioxide. Sodium chloride NaCl dissolves when water molecules continuously attack the NaCl crystal pulling away the individual sodium Na and chloride Cl ions. As a strong base sodium oxide also reacts with acids.
During the reaction the sodium metal may well become so hot that it catches fire and burns with a characteristic orange colour. Balance the equation Na2O. A concentrated solution of sodium oxide in water will have pH 14.
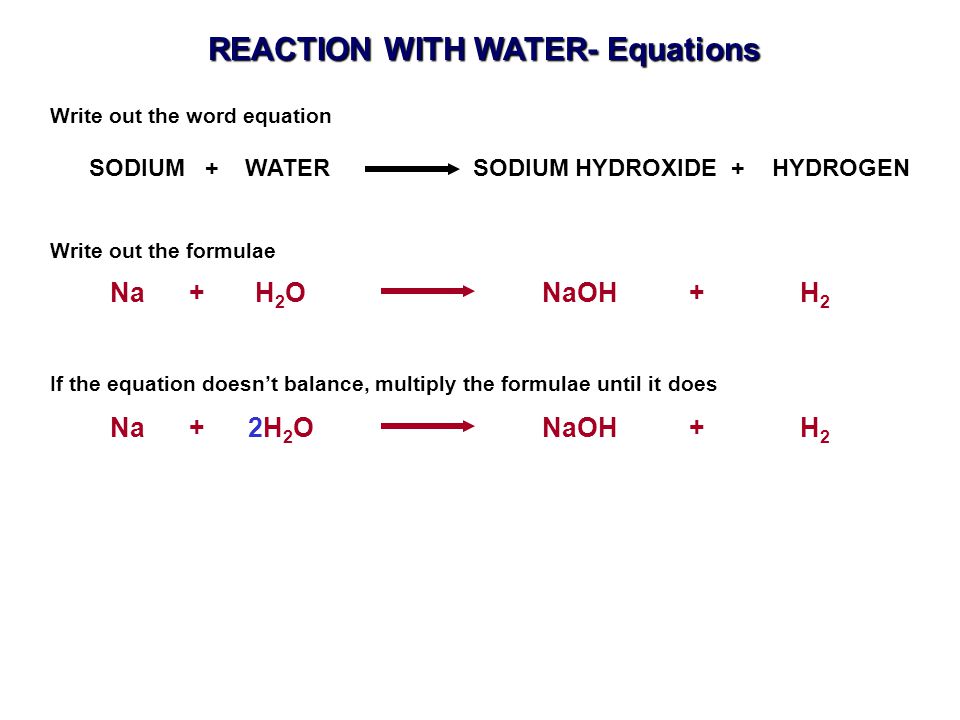
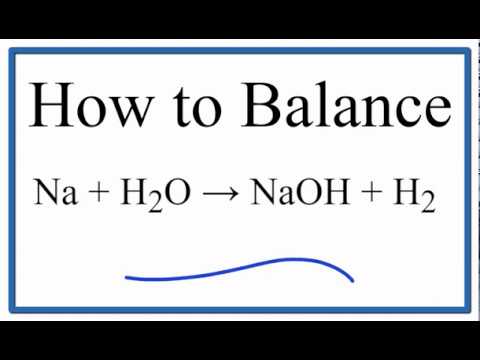
The word equation is. Reaction of sodium with water Sodium metal reacts rapidly with water to form a colourless solution of sodium hydroxide NaOH and hydrogen gas H2. 2Nas 2H2O 2NaOHaq H2g A colourless solution is formed consisting of strongly alkalic sodium hydroxide caustic soda and hydrogen gas.
To understand this process at the molecular level we must apply the three steps we previously discussed. The resulting solution is basic because of the dissolved hydroxide. Balanced Equation of sodium hydride and water 2NaH s 2H 2 O l 2NaOH aq H 2 g According to the stoichiometry 2 mol of NaH react with 2 mol of water and give two mole of NaOH and 1 mol of H 2.
Write balanced equations for the reaction of sodium potassium and calcium on cold water. Na2B4O710H2O or Na2 B4O5 OH48H2O this formula difference depending on the water content. 2Na 2H 2 O 2NaOH H 2 Sodium Cold water Sodium hydroxide Hydrogen gas.
Sodium reacts with water to for sodium hydroxide and hydrogen. The reaction is exothermic. Sodium metal reacts rapidly with water to form a colourless solution of sodium hydroxide NaOH and evolve hydrogen gas H2.