Smart Hard Unbalanced Chemical Equations

Chemical reactions have the same number of atoms before the reaction as after the reaction.
Hard unbalanced chemical equations. Ionic charges are not yet supported and will be ignored. So we must have 8HNO3and 8NO. The unbalanced chemical equation is C6H12O6 O2 CO2 H2O Step 2 Now algebraic variables are assigned to each species as stoichiometric coefficients in the unbalanced chemical equation.
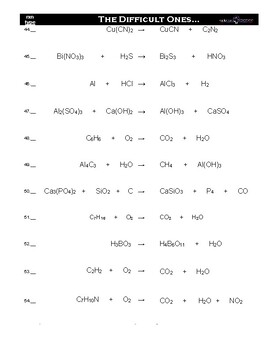
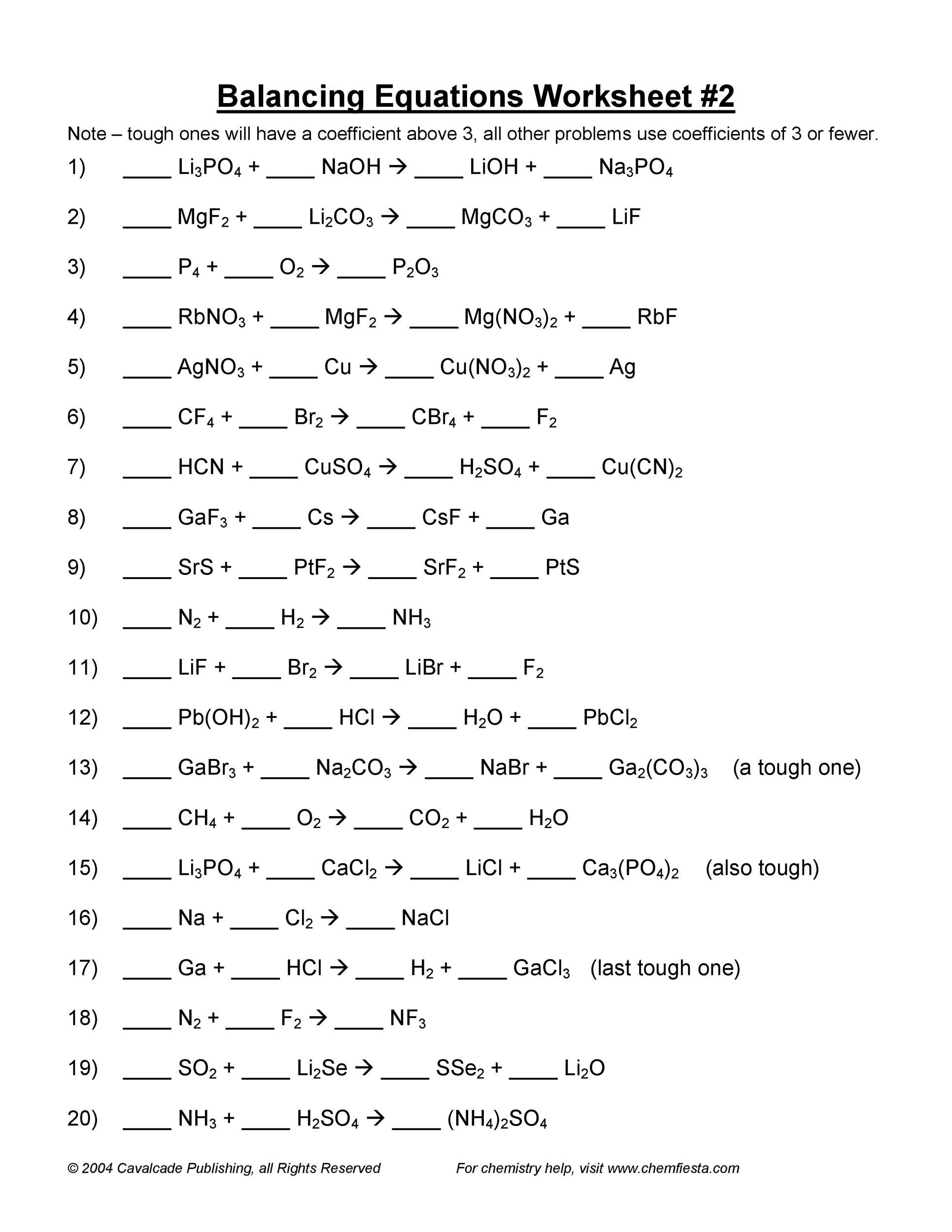
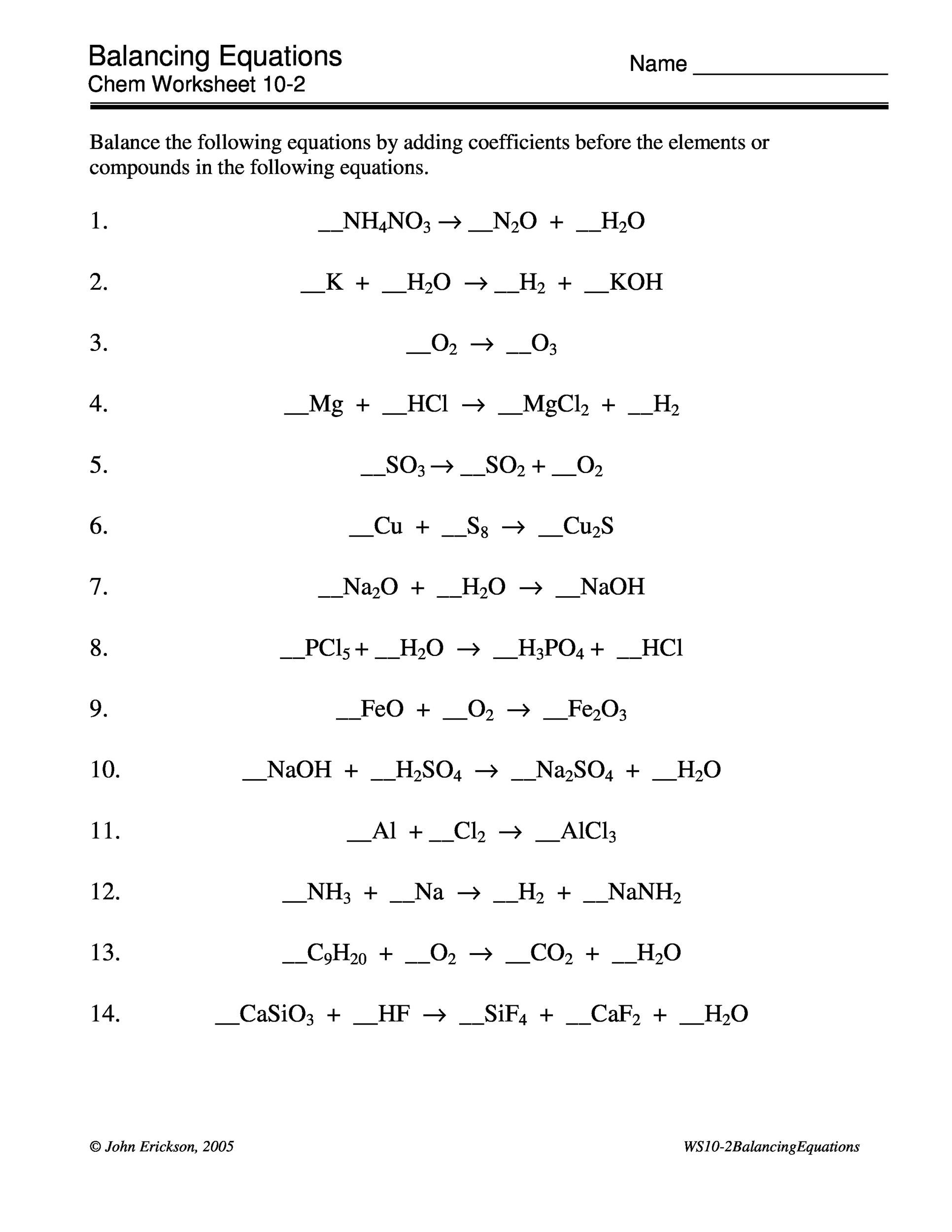
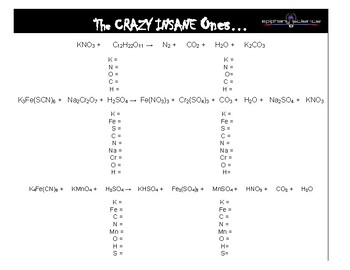
The following is a list of over 250 reaction equations that you may try to balance. In this example the equation can be written as follows. The chemical equation written by balancing total number of atoms of each element in reactants and products.
To balance a chemical equation enter an equation of a chemical reaction and press the Balance button. This video shows you how to balance equations that contain polyatomic ions that stay intact in the reactants and products. Please do not expect the link to the video I mention in the last p.
It contains plenty of examples and practice problems. This chemistry video tutorial explains the process of balancing chemical equations in chemistry. Caracterdesign Getty Images Great job.
Some tips on how to balance more complicated reactionsWatch the next lesson. Balancing Chemical Equations Problems 1 - 10. With the help of this application you will be easily able to balance the toughest of chemical equations.
YouTube wont let me edit to shorten the video or if its there I cant find it. In this equation chloride ion leaves Barium and attaches to sodium. If youre feeling a bit shaky on all the steps and details you can review the simple method of balancing equationsOtherwise you may wish to review how to balance oxidation-reduction or redox reactions or move on to understanding mole relations in balanced equations.