Cool Complete Combustion Of Coal

When coal combustion occurs in an environment low on.
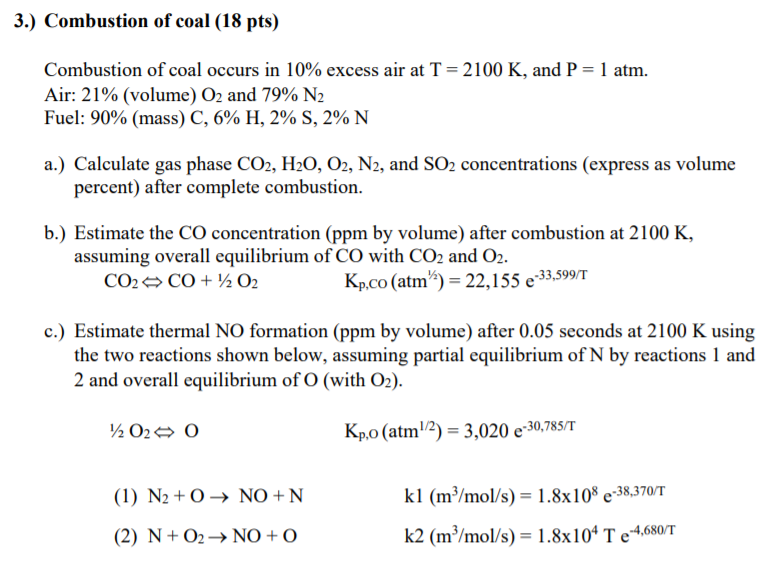
Complete combustion of coal. A complete combustion of 1000 kg of coal with 750 kg of C leads to 27 tons of CO 2 An incomplete combustion of 1000 kg of coal with 750 kg of C leads to 13 tons of CO. Thermogravimetry is applied to analyze the combustion characteristics and combustion kinetic mechanisms of three kinds of stone coal and the classify attribute of stone coal is investigated. The chemical equation for the combustion of coal is C O2 CO2.
Air supplied excess of 30 of that required for complete combustion of all carbon loaded. Due to the incomplete combustion under the surface coal fires in the underground produce more of these gases then a complete combustion of coal at the surface. The coal has the following composition.
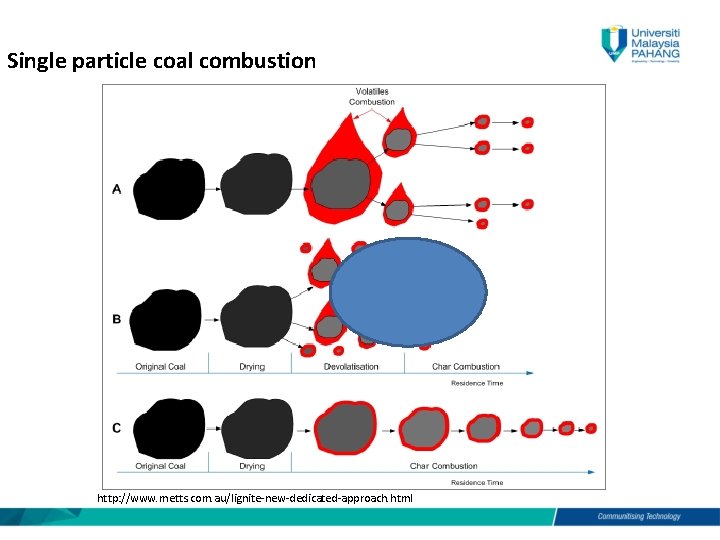
Coal combustion for domestic heating has been practiced sinceantiquity. Calculate the composition of the volume of flue gas that comes out. Coal combustion technology has been further developed since the late nineteenth century.
Therefore C kg of carbon will require C x 83 kg of oxygen which is equivalent to 266C kg of. If 97 of the carbon dioxide is complete and the rest is CO then. Thus the emitted PM is primarily composed of inorganic ash residues.
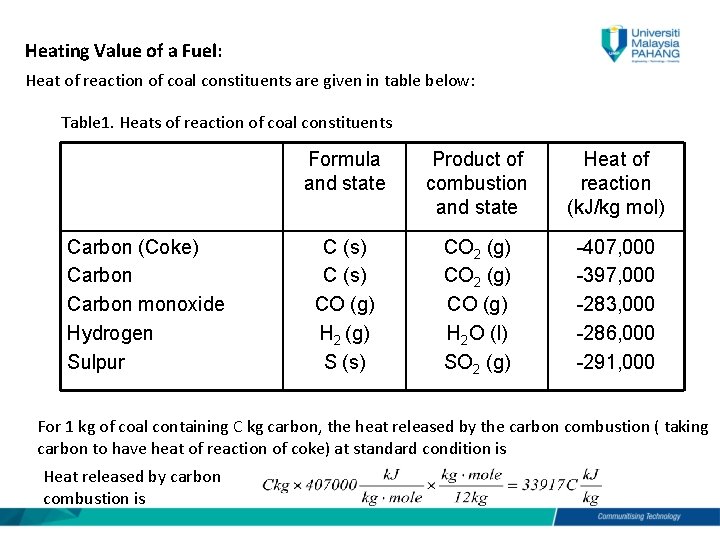
In this equation C represents the carbon in the coal which reacts with air represented by O2 to form carbon dioxide or CO2. Carbon 891 ash 109 Combustion chamber efficiency is 90 than loaded coal. Coal ash may either settle out in the boiler bottom ash or entrained in the flue gas.
In combustion oxygen may be supplied by using fresh air or a stream rich in oxygen to ensure complete combustion. Pulverised coal combustion technology is mainly used for steam generation in the power plant where most of the cases suspended coal bed is employed. In precombustion technologies combustion is made using pure oxygen up to 97 purity.